| Date | May 2015 | Marks available | 1 | Reference code | 15M.3.sl.TZ1.2 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Deduce | Question number | 2 | Adapted from | N/A |
Question
The structure of an unknown compound A with empirical formula CH2 can be determined using information from a variety of analytical techniques.
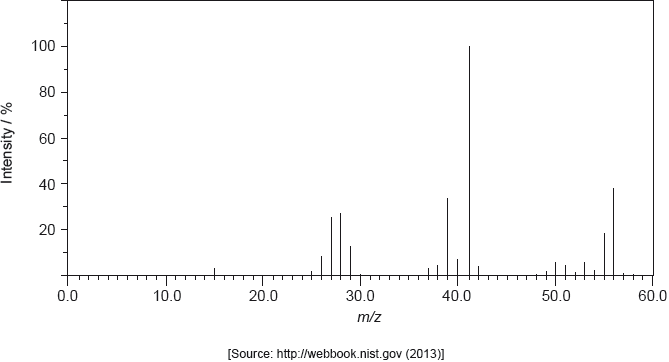
The mass spectrum of A is shown below.
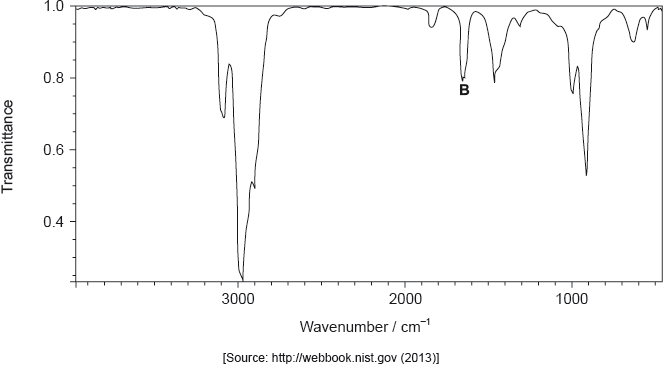
The infrared (IR) spectrum of A is shown below.
Determine the relative molecular mass of the compound from the mass spectrum and deduce the formula of the molecular ion.
Deduce the formulas of the fragments which give rise to peaks at \(m/z = 27\) and 29.
\(m/z = 27\):
\(m/z = 29\):
Identify the bond responsible for the IR absorption at B.
Deduce a structural formula consistent with the data.
Markscheme
56;
\({{\text{C}}_{\text{4}}}{\text{H}}_{\text{8}}^ + \);
Penalize missing charge only once in (i) and (ii).
\(m/z = 27:{{\text{C}}_{\text{2}}}{\text{H}}_3^ + /{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}^ + }/{\text{C}}{{\text{H}}_{\text{2}}}\)=\({\text{C}}{{\text{H}}^ + }\) and \(m/z = 29:{{\text{C}}_{\text{2}}}{\text{H}}_{\text{5}}^ + /{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH}}_{\text{2}}^ + {\text{;}}\)
Penalize missing charge only once in (i) and (ii).
C=C/carbon−carbon double bond;
Accept “alkenyl/alkene”.
CH3CH2CH=CH2;
Accept either a full or a condensed structural formula.
Examiners report
More than half of the candidates obtained the molecular mass from the spectrum. About a third of the candidates identified \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) as the molecular formula but only a few candidates remembered to include the positive charge on the molecular ion and scored the second mark.
About a third of the candidates identified the correct fragments. It was disappointing to see candidates suggesting fragments that did not match the masses of the peaks.
Very well answered.
Only a few candidates deduced the correct structural formula consistent with the data.

