| Date | November 2014 | Marks available | 3 | Reference code | 14N.3.hl.TZ0.1 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | State and Suggest | Question number | 1 | Adapted from | N/A |
Question
The mass spectrum and infrared (IR) spectrum of a compound are shown below.
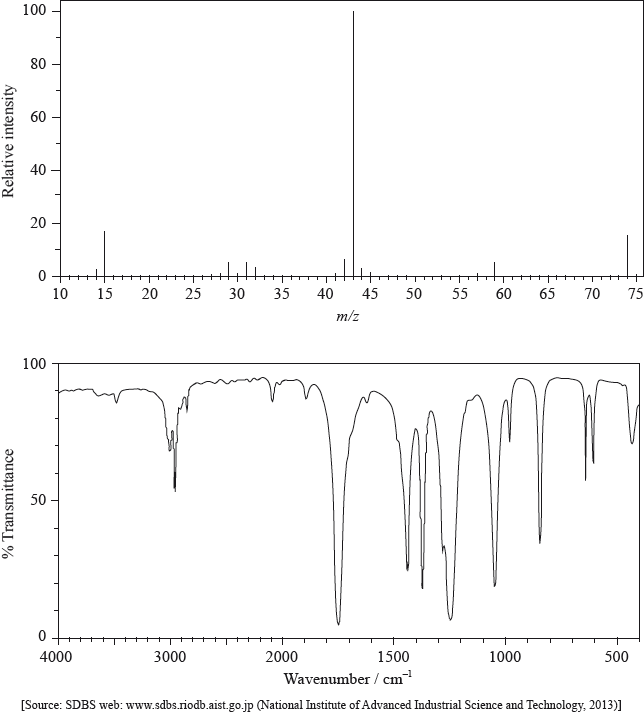
(i) State the information about this particular compound that can be derived from the mass spectrum and outline how it is found.
(ii) Suggest how the fragment with m/z = 43 is formed from the original molecule.
(i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ - 1}}\) to deduce one functional group that is present in the compound and one group that is absent.
Present:
Absent:
(ii) The molecular formula of the compound is \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\). Explain, with reference to another region of the IR spectrum, why the compound could not be propanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\).
(iii) Deduce the structures of two possible isomers of propanoic acid consistent with the IR spectrum.
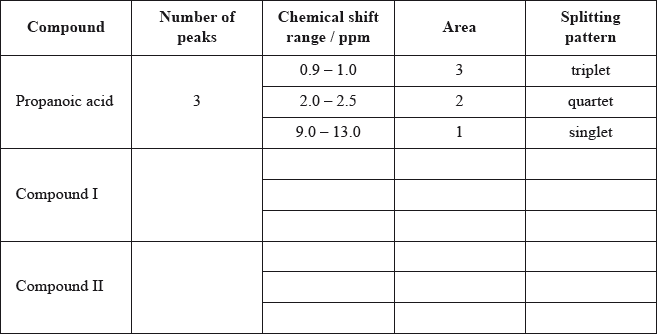
\(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy is often very useful in distinguishing between closely related compounds such as the ones shown below.
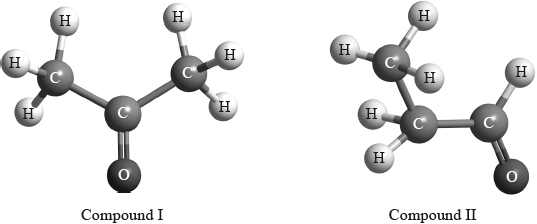
Deduce the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra you would expect for each compound and complete the table below. (The answers for propanoic acid are given as a guide.)
Note that some of the boxes may be blank.
Markscheme
(i) molar/molecular mass/\(M = 74{\text{ (g mo}}{{\text{l}}^{ - 1}}{\text{)}}\) / relative molecular mass/\({M_r} = 74\);
peak with highest m/z (ignoring any peak attributable to \(^{{\text{13}}}{\text{C}}\)) / found from parent/molecular ion peak;
Allow mass for m/z.
OR
compound has methyl/\({\text{C}}{{\text{H}}_{\text{3}}}\);
m/z = 15 due to \({\text{CH}}_3^ + \);
OR
compound has propyl/\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\)/isopropyl/\({\text{CH(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)/acetyl/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}\);
m/z = 43 due to \({{\text{C}}_{\text{3}}}{\text{H}}_7^ + {\text{/CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + {\text{/C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }\);
OR
compound has acetoxy/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}\);
m/z = 59 due to \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ + }\);
Fragment must contain + sign in relevant marks above.
Penalize missing charges where relevant once only in (a)(i) and (ii).
(ii) loss of \({\text{C}}{{\text{H}}_3}\)–O / loss of radical with m/z = 31 / formation of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }/{{\text{C}}_{\text{3}}}{\text{H}}_7^ + /{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + \);
Penalize missing charges where relevant once only in (a)(i) and (ii).
(i) Present: carbonyl/C=O;
Do not accept aldehyde / ketone.
Accept ester/alkanoate only if m/z = 59 given in (a)(i).
Absent: carbon–carbon double bond/C=C/alkene;
(ii) no (broad) absorption at 2500 – 3300 \({\text{(c}}{{\text{m}}^{ - 1}}{\text{)}}\);
no O–H bond;
Award [1 max] for just stating “absorption at 1050-1410 (cm–1) / C–O bond present of alcohol/ester/ether”.
Do not accept just “C–O bond present”.
Accept “peak” for absorption.
(iii) Any two structures from:
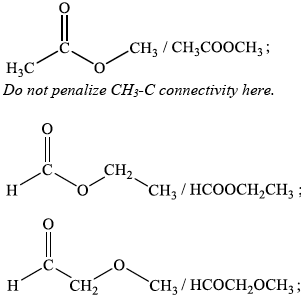
Award [1] for each 3 correct answers.
Award [5] for 13–14 correct answers.
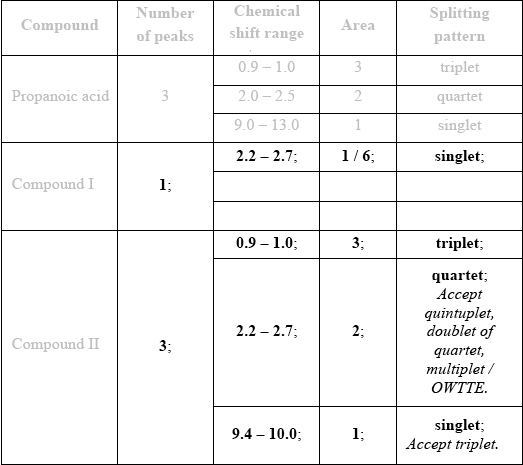
If more than one peak indicated for Compound I, then no “points”. Begin marking at Compound II and [3 max].
Examiners report
In general, most were able to deduce the molecular mass of the compound and how it is found. Unfortunately, some gave an account of the mechanism of fragmentation rather than giving information about this particular compound. Part (a) (i) asks about the mass spectrum; some candidates made the mistake of mentioning the IR spectrum at this point. It was encouraging to see the + sign normally included in any discussion of fragments in (a) (ii). The functional groups present and absent were usually identified correctly and most deduced why the compound could not be propanoic acid. Although, we allowed candidates to say that there was no OH bond present, they should be more careful to specify that it is the bond between the O and the H atoms that is not present. Care still needs to be taken in drawing structures, as in (b) (iii), to ensure the correct bond linkage. Many gave structures with an –OH group even though it had been ruled out in the previous part. Most were able to score well on the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) question although there were some inaccuracies in the chemical shifts suggested.
In general, most were able to deduce the molecular mass of the compound and how it is found. Unfortunately, some gave an account of the mechanism of fragmentation rather than giving information about this particular compound. Part (a) (i) asks about the mass spectrum; some candidates made the mistake of mentioning the IR spectrum at this point. It was encouraging to see the + sign normally included in any discussion of fragments in (a) (ii). The functional groups present and absent were usually identified correctly and most deduced why the compound could not be propanoic acid. Although, we allowed candidates to say that there was no OH bond present, they should be more careful to specify that it is the bond between the O and the H atoms that is not present. Care still needs to be taken in drawing structures, as in (b) (iii), to ensure the correct bond linkage. Many gave structures with an –OH group even though it had been ruled out in the previous part. Most were able to score well on the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) question although there were some inaccuracies in the chemical shifts suggested.
In general, most were able to deduce the molecular mass of the compound and how it is found. Unfortunately, some gave an account of the mechanism of fragmentation rather than giving information about this particular compound. Part (a) (i) asks about the mass spectrum; some candidates made the mistake of mentioning the IR spectrum at this point. It was encouraging to see the + sign normally included in any discussion of fragments in (a) (ii). The functional groups present and absent were usually identified correctly and most deduced why the compound could not be propanoic acid. Although, we allowed candidates to say that there was no OH bond present, they should be more careful to specify that it is the bond between the O and the H atoms that is not present. Care still needs to be taken in drawing structures, as in (b) (iii), to ensure the correct bond linkage. Many gave structures with an –OH group even though it had been ruled out in the previous part. Most were able to score well on the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) question although there were some inaccuracies in the chemical shifts suggested.

