DP Chemistry Questionbank

A.2 Metals and inductively coupled plasma (ICP) spectroscopy
Description
[N/A]Directly related questions
-
16N.3.sl.TZ0.4e:
Deduce the charge on the indium ion and the formula of indium sulfate.
-
16N.3.sl.TZ0.4a:
Calculate the charge, in coulombs, passed during the electrolysis.
-
16N.3.sl.TZ0.4b:
Calculate the amount, in mol, of electrons passed using section 2 of the data booklet.
-
16N.3.sl.TZ0.4c:
Calculate the mass of indium deposited by one mole of electrons.
-
16N.3.sl.TZ0.4d:
Calculate the number of moles of electrons required to deposit one mole of indium. Relative atomic mass of indium, Ar=114.82.
-
17M.3.sl.TZ1.7a:
State why lanthanum cannot be produced by reducing its oxide with carbon.
-
17M.3.sl.TZ1.7b:
Calculate the current (I), in A, required to produce 1.00 kg of lanthanum metal per hour. Use the formula and sections 2 and 6 of the data booklet.
-
17M.3.sl.TZ1.9c:
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.hl.TZ1.10a:
Outline the nature of the plasma state and how it is produced in ICP-MS.
-
17M.3.sl.TZ2.5c:
Another method of obtaining nickel is by electrolysis of a nickel(II) chloride solution. Calculate the mass of nickel, in g, obtained by passing a current of 2.50 A through the solution for exactly 1 hour. Charge (Q) = current (I) × time (t).
-
17M.3.sl.TZ2.5b.i:
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
-
17M.3.sl.TZ2.6b:
Metal impurities during the production of LCoS can be analysed using ICP-MS. Each metal has a detection limit below which the uncertainty of data is too high to be valid. Suggest one factor which might influence a detection limit in ICP-MS/ICP-OES.
-
17M.3.hl.TZ2.5c.i:
Rhodium is paramagnetic with an electron configuration of [Kr] 5s14d8.
Explain, in terms of electron spin pairing, why paramagnetic substances are attracted to a magnetic field and diamagnetic substances are not.
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
-
20N.3.sl.TZ0.4b(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020.
Write the half-equations for the reactions occurring in this electrolysis.
-
20N.3.sl.TZ0.4b(iv):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
-
20N.3.sl.TZ0.4b(iii):
Calculate the theoretical mass of magnesium obtained if a current of is used for hours. Use charge and section 2 of the data booklet
-
20N.3.hl.TZ0.4c(iii):
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
-
20N.3.hl.TZ0.4c(ii):
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020
Calculate the theoretical mass of magnesium obtained if a current of 3.00 A is used for hours. Use charge :(Q) = current (I) × time (t) and section 2 of the data booklet.
- 17N.3.sl.TZ0.4b.i: Outline why an alloy is usually harder than its components by referring to its structure.
-
17N.3.sl.TZ0.4c:
Explain how Inductively Coupled Plasma (ICP) Spectroscopy could be used to determine the concentration of mercury in a sample of dental filling.
-
18M.3.sl.TZ1.4c.ii:
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
-
18M.3.sl.TZ1.5:
Aluminium is produced by the electrolysis of a molten electrolyte containing bauxite.
Determine the mass, in g, of aluminium produced by the passage of a charge of 1.296 × 1013 C. Use sections 2 and 6 of the data booklet.
-
18M.3.sl.TZ2.3b:
ICP-MS is a reference mode for analysis. The following correlation graphs between ICP-OES and ICP-MS were produced for yttrium and nickel.
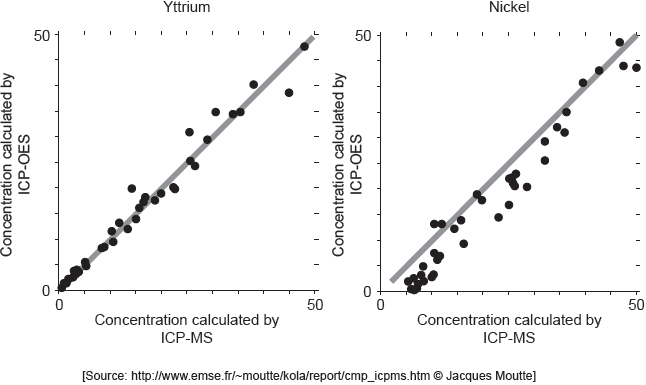
Each y-axis shows concentrations calculated by ICP-OES; each x-axis shows concentrations for the same sample as found by ICP-MS.
The line in each graph is y = x.
Discuss the effectiveness of ICP-OES for yttrium and nickel.
-
18M.3.sl.TZ2.3c.i:
Identify the purpose of each graph.
-
18M.3.sl.TZ2.3c.ii:
Calculate, to four significant figures, the concentration, in μg kg−1, of vanadium in oil giving a signal intensity of 14 950.
-
18N.3.sl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
-
18N.3.sl.TZ0.3b:
An unknown antacid sample has a lead ion concentration of 0.50 μg dm‒3.
Calculate the concentration of lead ions in the sample in mol dm‒3.
-
18N.3.hl.TZ0.3c:
Electrolysis is used to obtain lead from Pb2+ (aq) solution.
Determine the time, in hours, required to produce 0.0500 mol lead using a current (I) of 1.34 A. Use section 2 of the data booklet and the equation, charge (Q) = current (I) × time (t, in seconds).
- 18N.3.sl.TZ0.3a: State the type of particle present in the plasma formed.
-
18N.3.hl.TZ0.3b.i:
Calculate the concentration of lead ions in the sample in mol dm‒3.
- 18N.3.hl.TZ0.3a: State the type of particle present in the plasma formed.
-
19M.3.hl.TZ1.3b(i):
Identify the colour of the emission spectrum of lithium using section 17 of the data booklet.
-
19M.3.hl.TZ1.3b(ii):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.hl.TZ1.3b(iii):
Suggest a better method.
-
19M.3.hl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.hl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19M.3.hl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48 250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.sl.TZ1.3b(i):
Suggest why ICP-OES does not give good quantitative results for distinguishing 6Li from naturally occurring lithium.
-
19M.3.sl.TZ1.3b(ii):
Suggest a better method.
-
19M.3.sl.TZ1.3a(ii):
Explain why lithium is paramagnetic while lithium hydride is diamagnetic by referring to electron configurations.
-
19M.3.sl.TZ1.3c:
Lithium is obtained by electrolysis of molten lithium chloride. Calculate the time, in seconds, taken to deposit 0.694 g Li using a current of 2.00 A.
Q (charge) = I (current) × t (time)
-
19M.3.sl.TZ2.4a:
Determine the mass of aluminium, in g, that could be extracted from an appropriate solution by a charge of 48250 C. Use sections 2 and 6 of the data booklet.
-
19M.3.sl.TZ2.4b:
Once extracted, the purity of the metal can be assessed using ICP-MS. Suggest two advantages of using plasma technology rather than regular mass spectrometry.
-
19N.3.sl.TZ0.5a:
Discuss why different methods of reduction are needed to extract metals.
-
19N.3.sl.TZ0.5b(ii):
Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
