DP Chemistry Questionbank

C.6 Electrochemistry, rechargeable batteries and fuel cells (HL only)
Description
[N/A]Directly related questions
-
16N.3.hl.TZ0.21a:
The Geobacter species of bacteria can be used in microbial fuel cells to oxidise aqueous ethanoate ions,
CH3COO−(aq), to carbon dioxide gas.State the half-equations for the reactions at both electrodes.
-
16N.3.hl.TZ0.21b:
A concentration cell is an example of an electrochemical cell.
(i) State the difference between a concentration cell and a standard voltaic cell.
(ii) The overall redox equation and the standard cell potential for a voltaic cell are:
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθcell = +1.10 V
Determine the cell potential E at 298 K to three significant figures given the following concentrations in mol dm−3:
[Zn2+] = 1.00 × 10−4 [Cu2+] = 1.00 × 10−1
Use sections 1 and 2 of the data booklet.
(iii) Deduce, giving your reason, whether the reaction in (b) (ii) is more or less spontaneous than in the standard cell.
-
17M.3.hl.TZ1.22b.i:
Suggest a way in which they are similar.
-
17M.3.hl.TZ1.22b.ii:
Outline the difference between primary and rechargeable cells.
-
17M.3.hl.TZ1.22a:
Deduce half-equations for the reactions at the two electrodes and hence the equation for the overall reaction.
-
17M.3.hl.TZ1.22c:
Identify one factor that affects the voltage of a cell and a different factor that affects the current it can deliver.
-
17M.3.hl.TZ2.17c.iii:
Explain how the flow of ions allows for the operation of the fuel cell.
-
17M.3.hl.TZ2.17c.i:
Deduce the half-cell equations occurring at each electrode during discharge.
-
17M.3.hl.TZ2.17c.ii:
Outline the function of the proton-exchange membrane (PEM) in the fuel cell.
-
17N.3.hl.TZ0.20b:
Outline one advantage and one disadvantage of the methanol cell (DMFC) compared with a hydrogen-oxygen fuel cell.
-
17N.3.hl.TZ0.20a:
Deduce the half-equations and the overall equation for the reactions taking place in a direct methanol fuel cell (DMFC) under acidic conditions.
-
18M.3.hl.TZ1.14a.i:
Complete the half-equations on the diagram and identify the species moving between the electrodes.
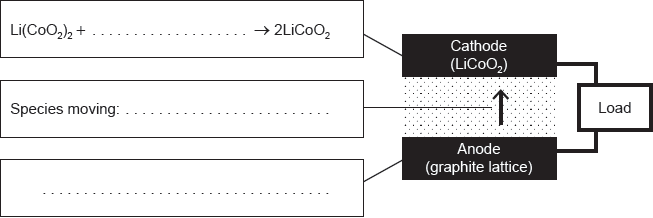
-
18M.3.hl.TZ1.14a.ii:
State the factor that limits the maximum current that can be drawn from this cell and how electrodes are designed to maximize the current.
-
18M.3.hl.TZ2.13c:
Fuel cells have a higher thermodynamic efficiency than octane. The following table gives some information on a direct methanol fuel cell.

Determine the thermodynamic efficiency of a methanol fuel cell operating at 0.576 V.
Use sections 1 and 2 of the data booklet.
-
18N.3.hl.TZ0.15c:
A voltaic cell consists of a nickel electrode in 1.0 mol dm−3 Ni2+ (aq) solution and a cadmium electrode in a Cd2+ (aq) solution of unknown concentration.
Cd (s) + Ni2+ (aq) → Cd2+ (aq) + Ni (s) EΘcell = 0.14 V
Determine the concentration of the Cd2+ (aq) solution if the cell voltage, E, is 0.19 V at 298 K. Use section 1 of the data booklet.
-
18N.3.hl.TZ0.15b:
Formulate half-equations for the reactions at the anode (negative electrode) and cathode (positive electrode) during discharge of a lithium-ion battery.
- 18N.3.hl.TZ0.15a: Outline how a rechargeable battery differs from a primary cell.
-
19M.3.hl.TZ1.17b(i):
Ethanol can be used in a direct-ethanol fuel cell (DEFC) as illustrated by the flow chart.
Deduce the half-equations occurring at electrodes A and B.
Electrode A:
Electrode B:
-
19M.3.hl.TZ1.17b(ii):
State the name and function of X in the diagram in (b)(i).
Name:
Function:
-
19M.3.hl.TZ1.17b(iii):
Outline why aqueous ethanol, rather than pure ethanol, is used in a DEFC.
-
19M.3.hl.TZ2.19a:
Outline how a microbial fuel cell produces an electric current from glucose.
C6H12O6 (aq) + 6O2 (g) → 6CO2 (g) + 6H2O (l)
- 19M.3.hl.TZ2.19c: Outline one difference between a primary and a secondary cell.
-
19M.3.hl.TZ2.19b:
The cell potential for the spontaneous reaction when standard magnesium and silver half-cells are connected is +3.17 V.
Determine the cell potential at 298 K when:
[Mg2+] = 0.0500 mol dm−3
[Ag+] = 0.100 mol dm−3Use sections 1 and 2 of the data booklet.
-
19N.3.hl.TZ0.20b(ii):
Suggest how PEM fuel cells can be used to produce a larger voltage than that calculated in (b)(i).
-
19N.3.hl.TZ0.20a:
Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):Cathode (positive electrode):
- 19N.3.hl.TZ0.20c: Suggest an advantage of the PEM fuel cell over the lead-acid battery for use in cars.
-
19N.3.hl.TZ0.20b(i):
Calculate the cell potential, Eθ, in V, using section 24 of the data booklet.
