| Date | November 2010 | Marks available | 6 | Reference code | 10N.2.sl.TZ0.2 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Deduce, Explain, Outline, and State | Question number | 2 | Adapted from | N/A |
Question
The alkenes are an example of a homologous series.
Bromine water, Br2(aq), can be used to distinguish between the alkanes and the alkenes.
The polymerization of the alkenes is one of the most significant reactions of the twentieth century.
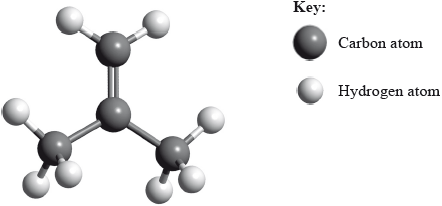
State the name of the alkene shown.
Bromine water, Br2(aq), can be used to distinguish between the alkanes and the alkenes.
(i) Describe the colour change observed when the alkene shown in part (a) is added to bromine water.
(ii) Draw the structural formula and state the name of the product formed.
(i) Outline two reasons why the polymers of the alkenes are of economic importance.
(ii) State the type of polymerization reaction shown by the alkene in part (a).
(iii) Deduce the structure of the resulting polymer showing three repeating units.
(iv) Explain why monomers are often gases or volatile liquids, but polymers are solids.
Markscheme
methylpropene;
Accept 2-methylpropene.
(i) brown/orange/yellow to colourless / bromine is decolorised;
(ii) 1,2-dibromo-2-methylpropane / 1,2-dibromomethylpropane / 1-bromo-2-methylpropan-2-ol / 1-bromomethylpropan-2-ol;
Do not penalize missing commas, hyphens or added spaces.
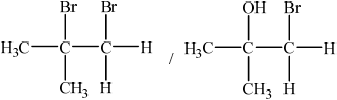 ;
;
Award [1] if structure and correct name are given for 2-bromo-2-methylpropan-1-ol
(i) synthesis of materials not naturally available/plastics;
chemically unreactive materials produced;
wide range of uses/physical properties / versatile;
cheap;
large industry;
uses a limited natural resource;
Award [2] for any two.
(ii) addition;
(iii) 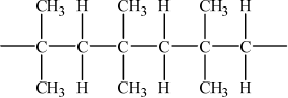 ;
;
Must show continuation bonds.
Ignore bracket around the 6 carbons.
Must have 6 carbons joined to each other along chain.
(iv) monomers are smaller molecules / have smaller surface area than polymers;
Accept monomers have lower molecular mass.
with weaker intermolecular/Van der Waals’/London/dispersion forces;
Accept opposite argument for polymers.
Examiners report
This question was generally well answered and many high scores were seen. Naming the initial compound was generally well done though many stated methylprop-1-ene which is incorrect.
Most could describe the colour change with bromine correctly though some incorrectly used ‘clear’ instead of ‘colourless’ and many could draw the structure of the dibromoalkane formed. Most candidates stated that plastics were produced from alkenes and suggested versatility or low cost and so were able to score full marks.
Addition polymerisation was well recalled but a large number of candidates made mistakes with the structure of the polymer, a surprisingly large number had bromine appearing attached to the carbon chain. Most understood that larger molar mass was why polymers had higher boiling points than monomers but not all correctly attributed it to stronger van der Waals‟ forces between molecules.

