| Date | November 2012 | Marks available | 2 | Reference code | 12N.2.hl.TZ0.1 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Identify and State | Question number | 1 | Adapted from | N/A |
Question
Two groups of students (Group A and Group B) carried out a project* on the chemistry of some group 7 elements (the halogens) and their compounds.
* Adapted from J Derek Woollins, (2009), Inorganic Experiments and Open University, (2008), Exploring the Molecular World.
In this project the students explored several aspects of the chemistry of the halogens. In the original preparation of ICl(l), they observed the yellow-green colour of chlorine gas, Cl2(g), reacting with solid iodine, I2(s).
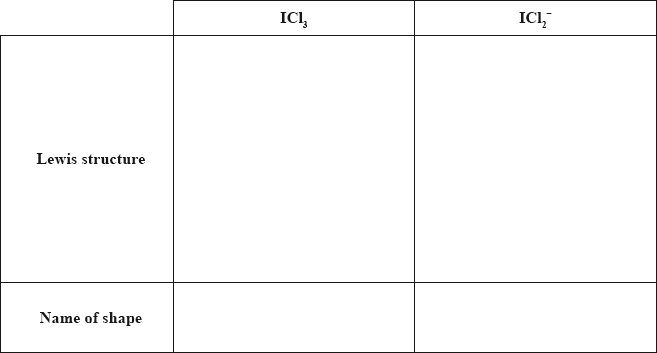
When iodine reacts with excess chlorine, \({\text{IC}}{{\text{l}}_{\text{3}}}\) can form. Deduce the Lewis (electron dot) structure of \({\text{IC}}{{\text{l}}_{\text{3}}}\) and \({\text{ICl}}_2^ - \) and state the name of the shape of each species.
State the full electron configuration of iodine \((Z = 53)\).
One important use of chlorine is in the synthesis of poly(chloroethene), PVC. Identify the monomer used to make PVC and state one of the uses of PVC.
Monomer:
Use:
Markscheme
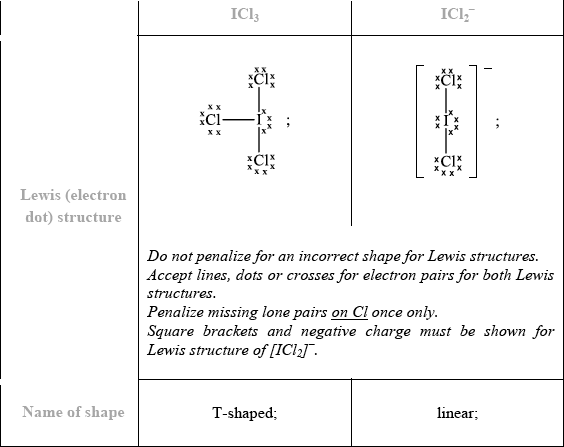
No ECF for shape if Lewis structure is incorrect.
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{p}}^{\text{6}}}{\text{5}}{{\text{s}}^{\text{2}}}{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{p}}^{\text{5}}}{\text{/1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{s}}^{\text{2}}}{\text{4}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{s}}^{\text{2}}}{\text{5}}{{\text{p}}^{\text{5}}}\);
No mark for 2,8,18,18,7 or [Kr] 5s24d105p5.
Allow electron configurations with order of sublevels interchanged.
Electrons must be represented as superscript to award mark.
Monomer:
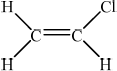 / chloroethene /\({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHCl}}\);
/ chloroethene /\({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHCl}}\);
Accept vinyl chloride or chloroethylene.
Allow C2H3Cl.
Use:
raincoats / packaging / window frames / pipes / carpets / gutters / electrical cable sheathing / covers for electrical wires / rope / bottles;
Accept suitable alternatives.
Do not allow glue.
Do not allow just plastic(s) or just windows.
Allow plastic bag.
Examiners report
Part (e) was by far one of the most disappointing questions on the entire paper with only the top-end candidates scoring all four marks. Many mistakes were seen, such as the usual mistakes of omitting lone pairs on terminal atoms and not including square brackets and the negative charge for the Lewis structure of the anion. The biggest problem however for candidates was failing to realise that for Lewis structures based on five negative charge centres or five electron domains, the lone pairs are inserted in the equatorial position and not the axial position, resulting in a T-shaped molecular geometry for \({\text{IC}}{{\text{l}}_{\text{3}}}\) and a linear shape for \({\text{ICl}}_2^ - \). Candidates may benefit in class from a careful discussion of the various angles resulting from LP-LP, LP-BP and BP-BP repulsions for such structures emanating from five electron domains. As a result of poor comprehension of this aspect of VSEPR Theory, a common incorrect molecular geometry of trigonal planar was often cited for the molecular geometry for\({\text{IC}}{{\text{l}}_{\text{3}}}\).
In part (f), the better candidates gave the correct full electron configuration for iodine. Surprisingly some of the weaker candidates gave electron arrangements which scored no marks and a few candidates gave rather sloppy configurations, either putting subscripts instead of superscripts or not putting the number of electrons as superscripts, which was rather disconcerting to see at HL.
In part (iii), a large number of candidates stated chloroethane instead of chloroethene for the monomer. Plastic was often given as a use of PVC. This however was not allowed for M2 and a more precise answer was required.

