| Date | November 2009 | Marks available | 3 | Reference code | 09N.2.sl.TZ0.4 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Predict and Suggest | Question number | 4 | Adapted from | N/A |
Question
The boiling points of the isomers of pentane, \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\), shown are 10, 28 and 36 °C, but not necessarily in that order.
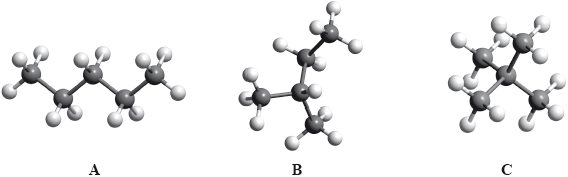
Identify the boiling points for each of the isomers A, B and C and state a reason for your answer.

State the IUPAC names of isomers B and C.
B:
C:
Both \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) and \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) can be used as fuels. Predict which compound would release a greater amount of heat per gram when it undergoes complete combustion. Suggest two reasons to support your prediction.
In many cities around the world, public transport vehicles use diesel, a liquid hydrocarbon fuel, which often contains sulfur impurities and undergoes incomplete combustion. All public transport vehicles in New Delhi, India, have been converted to use compressed natural gas (CNG) as fuel. Suggest two ways in which this improves air quality, giving a reason for your answer.
Markscheme
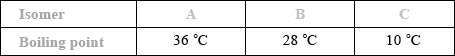
Award [1] if correct boiling points are assigned to 3 isomers.
increase in branching / more side chains / more spherical shape / reduced surface contact / less closely packed;
weaker intermolecular force/van der Waals’/London/dispersion forces;
Accept the opposite arguments
B: 2-methylbutane/methylbutane;
C: 2,2-dimethyl propane/dimethyl propane;
Do not penalize missing commas, hyphens or added spaces.
Do not accept 2-dimethylpropane, or 2,2-methylpropane.
\({{\text{C}}_5}{{\text{H}}_{12}}\);
Accept any two of the following explanations.
\({{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}}\) has greater molar mass / produces less grams of \({\text{C}}{{\text{O}}_2}\) and \({{\text{H}}_2}{\text{O}}\) per gram of the compound / suitable calculations to show this;
\({{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}}\) contains an O atom which contributes nothing to the energy released / partially oxidized / OWTTE;
analogous compounds such as butane and butan-1-ol show a lower value for the alcohol per mole in the data book / OWTTE;
the total bond strength in the pentanol molecule is higher than the total bond strength in pentane;
the total amount of energy produced in bond formation of the products per mole is the same;
fewer moles of pentanol in 1 g;
pentanol requires more energy to break intermolecular forces/hydrogen bonding / OWTTE;
Improvements [2]
less/no particulates/C/CO/VOC’s produced with CNG;
less/no SO2/SOx produced;
Reasons [1 max]
CO/SO2 toxic/poisonous;
SO2 causes acid rain;
CNG is likely to undergo complete/more combustion;
CNG has no/less sulfur impurities;
Examiners report
This question also featured on the G2 forms, as some teachers thought that the inclusion of Aim 8 type questions such as this would disadvantage candidates. However performance by the majority was very good. It should be noted that questions of this type will always be asked in future papers. In (a), most candidates correctly identified the boiling points although some reversed the order and a few had B with the highest boiling point. Explanations for this trend were not so well answered. Some candidates referred to breaking bonds in the carbon chain and several answers referred to the length of the carbon chain rather than the degree of branching.
The IUPAC names were generally well known, with the most common errors being the use of “pent” instead of “prop” and the omission of one of the locants, or “di” in “2,2-dimethylpropane”.
Many candidates scored 0 in part b) as they incorrectly suggested that pentan-1-ol would have a larger energy density than pentane. It is clear from the variety of wrong answers and reasons that candidates are not familiar with the ideas tested in this question. Many candidates referred to hydrogen bonds between molecules, as a reason for pentan-1-ol releasing more energy, only a few consulted their Data Booklet and made reference to this.
In c) there were 2 marks for improvements to air quality and 1 mark for a reason. Most candidates included the idea that there would be less carbon monoxide formed and that this was a poisonous gas. There were fewer references to oxides of sulfur, although many said that CNG has fewer S impurities rather than to say that less SO2/SOx is released, in this case as they had already scored their explanation mark they could not score for this and ended up with 2 marks out of 3. Some candidates did not centre their answer on what was being asked. Also, some candidates said that natural gas is a natural fuel while diesel is not, and that natural gas, when it burns does not produce carbon dioxide.

