| Date | May 2015 | Marks available | 2 | Reference code | 15M.2.hl.TZ1.5 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | State | Question number | 5 | Adapted from | N/A |
Question
Ethanol has many industrial uses.
State an equation for the formation of ethanol from ethene and the necessary reaction conditions.
Equation:
Conditions:
Define the term average bond enthalpy.
Ethanol can be used as a fuel. Determine the enthalpy of combustion of ethanol at 298 K, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), using the values in table 10 of the data booklet, assuming all reactants and products are gaseous.
Students can also measure the enthalpy of combustion of ethanol in the laboratory using calorimetry. Suggest the major source of systematic error in these procedures.
State the equation for the acid-catalysed reaction of ethanol with propanoic acid and state the name of the organic product.
Equation:
Name of the organic product:
A polyester can be formed when ethane-1,2-diol reacts with benzene-1,4-dicarboxylic acid.
Deduce the structure of the repeating unit and state the other product formed.
Repeating unit:
Other product:
State the type of polymerization that occurs.
The standard enthalpy change of combustion, \(\Delta H_{\text{c}}^\Theta \), of propanoic acid is \( - 1527{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine the standard enthalpy change of formation of propanoic acid, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using this information and data from table 12 of the data booklet.
Deduce, giving a reason, the sign of the standard entropy change of the system for the formation of propanoic acid from its elements.
Identify three allotropes of carbon and describe their structures.
Markscheme
Equation:
\({\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH/}}{{\text{C}}_2}{{\text{H}}_4} + {{\text{H}}_2}{\text{O}} \to {{\text{C}}_2}{{\text{H}}_5}{\text{OH}}\);
Conditions:
(concentrated) sulfuric acid/\({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\);
Do not accept dilute sulfuric acid.
Accept phosphoric acid/\({H_3}P{O_4}\) (on pellets of silicon dioxide) (for industrial preparation).
heat / high temperature;
Do not accept warm.
Accept high pressure (for industrial preparation) for M3 only if \({H_3}P{O_4}\) is given for M2.
energy needed to break (1 mol of) a bond in the gaseous state/phase;
(averaged over) similar compounds;
Do not accept “similar bonds” instead of “similar compounds”.
Concept of “similar” is important for M2.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{3}}{{\text{O}}_2} \to {\text{2C}}{{\text{O}}_2} + {\text{3}}{{\text{H}}_2}{\text{O}}\);
Bonds broken:
\(347 + (5 \times 413) + 358 + 464 + (3 \times 498)/4728{\text{ (kJ)}}/{\text{C–C}} + 5{\text{C–H}} + {\text{C–O}} + {\text{O–H}} + {\text{3O=O}}\);
Bonds made:
\((4 \times 746) + (6 \times 464) = 5768{\text{ (kJ)}}/{\text{4C = O}} + {\text{6O–H}}\);
\(\Delta H = (4728 - 5768 = ) - 1040{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\) / bonds broken − bonds formed;
Award [4] for correct final answer.
Award [3] for (+)1040 (\(kJ\,mo{l^{ - 1}}\)).
heat loss (to the surroundings);
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OOCCH2C}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}}\);
ethyl propanoate;
Do not penalize if equilibrium arrow missing.
Repeating unit:
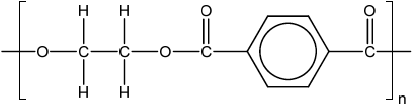 ;
;
Continuation lines must be shown.
Ignore brackets and n.
Accept condensed formulas such as \(C{H_2}\) and \({C_6}{H_4}\).
Other product:
\({{\text{H}}_{\text{2}}}{\text{O}}\)/water;
condensation;
\({\text{3C(s)}} + {\text{3}}{{\text{H}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH(l)}}\);
\(\Delta H_{\text{f}}^\Theta = \sum \Delta H_{\text{c}}^\Theta {\text{ (reactants)}} - \sum {\Delta H_{\text{c}}^\Theta {\text{ (products)}}} \);
Accept any suitable energy cycle.
\(\sum {\Delta H_{\text{c}}^\Theta {\text{ (reactants)}}} = 3 \times ( - 394) + 3 \times ( - 286)/ - 2040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
\((\Delta H_{\text{f}}^\Theta = [3 \times ( - 394) + 3 \times ( - 286)] - ( - 1527) = ) - 513{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
OR
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH(l)}} + {\text{3.5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(g)}}\);
\(\Delta H_{\text{c}}^\Theta = \sum {\Delta H_{\text{f}}^\Theta {\text{ }}(products)} - \sum {\Delta H_{\text{f}}^\Theta {\text{ }}(reactants)} \);
\(\sum {\Delta H_{\text{f}}^\Theta {\text{ (products)}}} = 3 \times ( - 394) + 3 \times ( - 286)/ - 2040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\({\text{(}}\Delta H_{\text{f}}^\Theta = [3 \times ( - 394) + 3 \times ( - 286)] - ( - 1527) = ) - 513{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Ignore state symbols.
Award [4] for correct final answer.
negative;
reduction in the number of gaseous molecules;
Allotropes:
Any three allotropes for [1] from:
diamond
graphite
fullerene
graphene;
Allow (carbon) nanotubes for graphene.
Accept \({C_{{\text{60}}}}\)/\({C_{{\text{70}}}}\)/buckminsterfullerene/bucky balls for fullerene.
Structures:
Any three for [3] from:
Diamond:
tetrahedral arrangement of (carbon) atoms/each carbon bonded to four others / \({\text{s}}{{\text{p}}^{\text{3}}}\) and 3D/covalent network structure;
Graphite:
each carbon bonded to three others (in a trigonal planar arrangement) / \({\text{s}}{{\text{p}}^{\text{2}}}\) and 2D / layers of (carbon) atoms;
Fullerene:
each (carbon) atom bonded to three others (in a trigonal arrangement) / \({\text{s}}{{\text{p}}^{\text{2}}}\) and joined in a ball/cage/sphere/connected hexagons and pentagons;
Accept “trigonal planar” for “each carbon atom bonded to three others” part in M4.
Graphene:
each carbon bonded to three others (in a trigonal arrangement) / \({\text{s}}{{\text{p}}^{\text{2}}}\) and 2D structure;
Examiners report
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.
There was poor understanding of the transformation in (a). When defining the average bond enthalpy in (b), the notion of “gaseous” was frequently omitted and very few mentioned the bonds being in similar compounds. In the calculation, many omitted the C–C bond and many did not work from a properly balanced equation which led to disaster. Nearly every candidate attempting this question was able to suggest “heat loss”. In (d) the usual errors were made; the name was the wrong way round, water was missing from the equation and wrong products (such as pentanoic acid) were suggested. In (e) (i) the diagrams were poor but water was usually given correctly. Most gave condensation as the type of polymerization. The key to gaining marks in questions such as (f) (i) is to start with a balanced equation, [1 mark], and then set the calculation out correctly and tidily. Part marks cannot be given if the examiner cannot follow what the candidate is doing. Many correctly gave “negative” in (ii) but the explanations lacked clarity. Most gained a mark in (g) for knowing three allotropes but the description of structures was poorly done. The [4] (marks) for this part gives some idea of the amount of detail expected.

