| Date | May 2011 | Marks available | 1 | Reference code | 11M.2.hl.TZ2.7 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Describe | Question number | 7 | Adapted from | N/A |
Question
The standard electrode potential for a half-cell made from iron metal in a solution of iron(II) ions, \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\), has the value \( - 0.45{\text{ V}}\).
Consider the following table of standard electrode potentials.
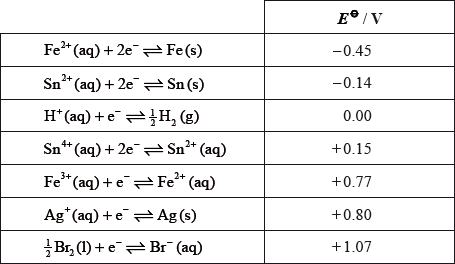
From the list above:
An acidified solution of potassium dichromate is often used as an oxidizing agent in organic chemistry. During the oxidation reaction of ethanol to ethanal the dichromate ion is reduced to chromium(III) ions according to the following unbalanced half-equation.
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to {\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
Sodium metal can be obtained by the electrolysis of molten sodium chloride.
Define standard electrode potential.
Explain the significance of the minus sign in \( - {\text{0.45 V}}\).
State the species which is the strongest oxidizing agent.
Deduce which species can reduce \({\text{S}}{{\text{n}}^{4 + }}{\text{(aq)}}\) to \({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) but will not reduce \({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) to Sn(s) under standard conditions.
Deduce which species can reduce \({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) to Sn(s) under standard conditions.
Draw a labelled diagram of a voltaic cell made from an Fe (s) / \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) half-cell connected to an Ag(s) / \({\text{A}}{{\text{g}}^ + }{\text{(aq)}}\) half-cell operating under standard conditions. In your diagram identify the positive electrode (cathode), the negative electrode (anode) and the direction of electron flow in the external circuit.
Deduce the equation for the chemical reaction occurring when the cell in part (c) (i) is operating under standard conditions and calculate the voltage produced by the cell.
Describe the colour change that will be observed in the reaction.
Deduce the oxidation number of chromium in \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_7^{2 - }\).
State the balanced half-equation for the reduction of dichromate ions to chromium(III) ions.
Deduce the half-equation for the oxidation of ethanol to ethanal and hence the overall redox equation for the oxidation of ethanol to ethanal by acidified dichromate ions.
Explain why it is necessary to carry out the reaction under acidic conditions.
Identify the organic product formed if excess potassium dichromate is used and the reaction is carried out under reflux.
Explain why it is very difficult to obtain sodium from sodium chloride by any other method.
Explain why an aqueous solution of sodium chloride cannot be used to obtain sodium metal by electrolysis.
Markscheme
the potential difference/voltage obtained when a half-cell is connected to a standard hydrogen electrode;
under standard conditions / \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solutions, 298 K;
the electrons flow from the half-cell to the standard hydrogen electrode / the half-cell forms the negative electrode when connected to the standard half-cell / Fe is a better reducing agent than \({{\text{H}}_2}\) / Fe is above \({{\text{H}}_2}\) in electrochemical series;
Accept “the half reaction is not spontaneous”.
bromine/ \({\text{B}}{{\text{r}}_2}\);
hydrogen/ \({{\text{H}}_2}\);
iron/Fe;
Ignore coefficients for Br2, H2 or Fe.
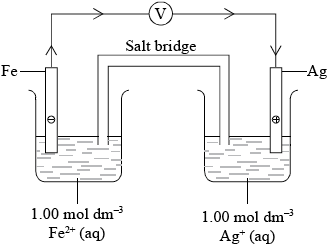
correct diagram including voltmeter;
No credit if wires to electrodes immersed in the solutions.
labelled salt bridge;
Do not accept name of salt (e.g. potassium nitrate) in place of salt bridge.
correctly labelled (+) and (–) electrodes / cathode and anode;
1 or \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) concentrations/298 K;
flow of electrons from Fe to Ag in external circuit;
Award [2 max] if battery shown instead of voltmeter.
\({\text{Fe}} + {\text{2A}}{{\text{g}}^ + } \to {\text{F}}{{\text{e}}^{2 + }} + {\text{2Ag}}\);
\(E_{{\text{cell}}}^\Theta {\text{ }}( = 0.80 - ( - 0.45) = ){\text{ }}1.25{\text{ V}}\);
Ignore state symbols.
(the solution changes) from orange to green;
+6;
Do not accept 6, 6+ or the use of Roman numerals unless they have already been penalized in (2)(a).
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{ + 14}}{{\text{H}}^ + } + {\text{6}}{{\text{e}}^ - } \to {\text{2C}}{{\text{r}}^{3 + }} + {\text{7}}{{\text{H}}_2}{\text{O}}\);
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} \to {\text{C}}{{\text{H}}_3}{\text{CHO}} + {\text{2}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - }\);
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - } + {\text{3C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{8}}{{\text{H}}^ + } \to {\text{2C}}{{\text{r}}^{3 + }} + {\text{3C}}{{\text{H}}_3}{\text{CHO}} + {\text{7}}{{\text{H}}_2}{\text{O}}\)
For second equation award [1] for correct reactants and products and [1] for correct balancing.
\({{\text{H}}^ + }\) is a reactant / OWTTE;
ethanoic acid / \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) / acid;
Accept acetic acid.
sodium is a very powerful reducing agent/high in electrochemical series;
any chemical reducing agent would need to be even higher in ECS to reduce \({\text{N}}{{\text{a}}^ + }\) / OWTTE;
\({{\text{H}}^ + }\) ions gain electrons more readily than \({\text{N}}{{\text{a}}^ + }\) / hydrogen is evolved instead;
hydrogen is below Na in ECS;
if sodium were to be formed it would react with the water in the solution / OWTTE;
Examiners report
This was the third most popular question. Most candidates were able to give only an incomplete definition of the standard electrode potential; the need for standard conditions was often omitted.
Only the strongest candidates were able to clearly explain the significance of the negative sign for the standard electrode potential of the half cell.
7(b) proved to be confusing for some candidates with many giving the half-equation instead of a specific species.
7(b) proved to be confusing for some candidates with many giving the half-equation instead of a specific species.
7(b) proved to be confusing for some candidates with many giving the half-equation instead of a specific species.
Labelling the voltaic cell was generally well done in (c) (i) but some responses mixed up the cathode and anode or gave a battery instead off a voltmeter. The most common omission, however, involved the concentrations (\({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)) of the solution and the temperature of 298 K.
A minority of candidates gave an equilibrium sign for the cell reaction and some candidates forgot the V units.
In (d) a surprising number of candidates were unable to give the colour change observed when dichromate(VI) ions are reduced to chromium(III) ions by ethanol.
The majority of candidates were not able to write the balanced redox reaction for the production of ethanal.
Most candidates were able to identify ethanoic acid as the product of further oxidation under reflux.
Many were unable to explain the need to carry out the reaction under acidic conditions.
The presence of \({{\text{H}}^ + }\) as a reactant in the equation was the expected response.
(e) proved to be very challenging with not many able to explain why it is difficult to obtain sodium from the electrolysis of aqueous sodium chloride.
All sorts of misunderstandings were in evidence, many involving a discussion of the compound‟s high lattice enthalpy.

