| Date | May 2014 | Marks available | 2 | Reference code | 14M.2.sl.TZ2.6 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Outline and State | Question number | 6 | Adapted from | N/A |
Question
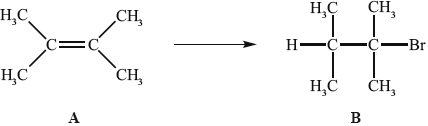
Alkenes, such as A (shown below), are important intermediates in the petrochemical industry because they undergo addition reactions to produce a wide variety of products, such as the conversion shown below.
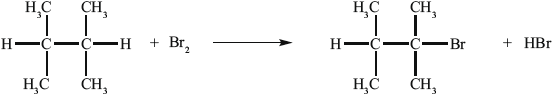
Another way to make B is the reaction shown below.
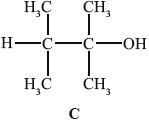
B can be converted into C.
In the gas phase, A reacts with hydrogen to form D.
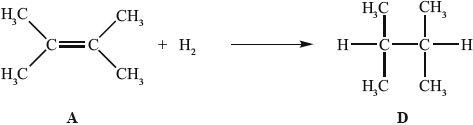
Applying IUPAC rules, state the name of A.
State the reagent required to convert A into B.
(i) State the conditions required for this reaction to occur.
(ii) Outline why it would give a poor yield of the desired product.
(i) State the reagent required.
(ii) Explain the mechanism of this reaction, using curly arrows to represent the movement of electron pairs.
A can also be converted into C without going via B. State the reagent and conditions required.
(i) State why C is not readily oxidized by acidified potassium dichromate(VI).
(ii) Deduce the structural formula of an isomer of C that could be oxidized to a carboxylic acid by this reagent.
State the conditions required for this reaction to occur.
State the homologous series to which D belongs.
Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of A with hydrogen, using Table 10 of the Data Booklet, and state whether the reaction is exothermic or endothermic.
The standard enthalpy change of combustion of A is \( - 4000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the amount of A, in mol, that would have to be burned to raise the temperature of \({\text{1 d}}{{\text{m}}^{\text{3}}}\) of water from 20 °C to 100 °C.
Markscheme
2,3-dimethylbut-2-ene;
Ignore punctuation.
hydrogen bromide / hydrobromic acid / HBr;
(i) ultraviolet light/sunlight;
Accept “very high temperature”.
(ii) random/further/multiple substitution (so low probability of desired product) / would give a mixture of many different products / OWTTE;
(i) (aqueous) sodium hydroxide/NaOH / potassium hydroxide/KOH;
Accept hydroxide ion/OH–.
(ii) 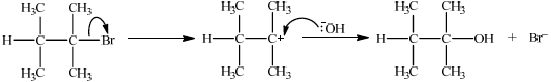
\({S_N}1\):
curly arrow from C–Br bond showing Br leaving;
representation of tertiary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
Do not allow arrow originating on H in HO–.
Award [2] for perfect SN2 mechanism.
Award [1] for SN2 mechanism with minor mistakes.
water / steam;
heat and acid catalyst /(concentrated) \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}/{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\);
(i) (it is a) tertiary/3° alcohol / carbon of C–OH is not bonded to a hydrogen;
Accept “it is not a primary or secondary alcohol”.
(ii) any \({{\text{C}}_6}{{\text{H}}_{14}}{\text{O}}\) primary alcohol / \({{\text{C}}_5}{{\text{H}}_{11}}{\text{C}}{{\text{H}}_2}{\text{OH}}\);
Ni/Pt/Pd catalyst;
alkanes;
bonds broken: (E(C=C) + E(H–H) = 612 + 436 =) \({\text{1048 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept (6956 + 436 =) 7392 if all bonds in alkene broken.
bonds formed: E(C–C) + 2 \( \times \) E(C–H) = 347 + (2 \( \times \) 413) = \({\text{1173 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept 7517 if all the bonds in the product are summed.
\(\Delta H = 1048 - 1173/7392 - 7517 = - 125{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for +125.
exothermic;
Apply ECF if sign of \(\Delta H\) incorrect.
Do not award a mark for “exothermic” if \(\Delta H\) given as positive.
energy required to heat water \(\left( { = m \times s \times \Delta T = 1 \times 4.18 \times (100 - 20)} \right) = 334.4{\text{ }}({\text{kJ}})\);
Ignore sign of energy change.
amount required \(\frac{{334.4}}{{4000}} = 0.0836{\text{ (mol)}}\);
Award [2] for correct final answer.
Examiners report
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.
Probably the most popular and successfully answered. Most students were family with IUPAC nomenclature and realised that UV radiation is required to initiate the halogenation of an alkane, though fewer realised that the much greater probability of forming a different isomer, or the problem of polysubstitution would result in a very low yield. The conditions for the hydrolysis of the bromoalkane were well known, though fewer recognised it as a tertiary halogenoalkane and described the \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction mechanism. Only a small number of candidates were able to show the electron pair originating from C–Br bond or the lone pair on the oxygen or negative charge of the hydroxide ion. Many candidates knew that tertiary alcohols could not be oxidised and correctly drew primary structures for alcohols that could be oxidised to carboxylic acids although some made careless errors and drew secondary structures or did not answer the question and proposed aldehydes. Many candidates were able to determine the enthalpy change, from bond enthalpies but some had not read the question carefully and did not address the final mark. A significant number of candidates made small errors but still gained ECF marks as they had set their working out clearly. The calculation of the amount of fuel required to raise the temperature proved more difficult with many students overlooking the volume of water and using the data to calculate the mass of the hydrocarbon that would be heated by 80 °C by the molar enthalpy of combustion and using the specific heat capacity of water.

