| Date | May 2014 | Marks available | 1 | Reference code | 14M.2.sl.TZ1.7 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Draw | Question number | 7 | Adapted from | N/A |
Question
\({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ethanoic acid were added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.150 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydrogencarbonate solution, \({\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\).
The molar mass of a volatile organic liquid, X, can be determined experimentally by allowing it to vaporize completely at a controlled temperature and pressure. 0.348 g of X was injected into a gas syringe maintained at a temperature of 90 °C and a pressure of \(1.01 \times {10^5}{\text{ Pa}}\). Once it had reached equilibrium, the gas volume was measured as \({\text{95.0 c}}{{\text{m}}^{\text{3}}}\).
Bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), undergoes a substitution reaction to form ethanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
Outline how electrical conductivity can be used to distinguish between a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), and a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrochloric acid, HCl.
(i) State an equation for the reaction of ethanoic acid with a solution of sodium hydrogencarbonate.
(ii) Determine which is the limiting reagent. Show your working.
(iii) Calculate the mass, in g, of carbon dioxide produced.
(i) Determine the amount, in mol, of X in the gas syringe.
(ii) Calculate the molar mass of X.
(i) Identify the reagent necessary for this reaction to occur.
(ii) Deduce the mechanism for the reaction using equations and curly arrows to represent the movement of electron pairs.
Determine the enthalpy change, in kJ mol\(^{ - 1}\), for this reaction, using Table 10 of the Data Booklet.
Bromoethene, \({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHBr}}\), can undergo polymerization. Draw a section of this polymer that contains six carbon atoms.
Markscheme
HCl is a strong acid and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) is a weak acid so HCl has higher conductivity / HCl dissociates completely in water and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) does not, so HCl has higher conductivity / HCl is stronger acid (than \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) so has higher \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and higher conductivity;
(i) \({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {\text{HCO}}_3^ - {\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Accept NaHCO3(aq) and CH3COONa (aq) instead of ions.
Ignore state symbols.
(ii) \(n{\text{(C}}{{\text{H}}_3}{\text{COOH)}} = 0.00500{\text{ (mol)}}\) and \(n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\({\text{NaHC}}{{\text{O}}_3}\) is limiting;
(iii) \(n{\text{(C}}{{\text{O}}_2}{\text{)}} = n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\(m{\text{(C}}{{\text{O}}_2}{\text{)}} = 0.00450 \times 44.01 = 0.198{\text{ (g)}}\);
Award [2] for correct final answer.
(i) \(T = 363{\text{ K}}\) and \(V = 9.50 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}\);
Accept V = 9.5 \( \times \) 10–2 dm3 if P is used as 101 kPa in calculation.
\(n = \frac{{PV}}{{RT}} = \frac{{1.01 \times {{10}^5} \times 9.50 \times {{10}^{ - 5}}}}{{8.31 \times 363}}\);
\( = 3.18 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [3] for correct final answer.
(ii) \(M = \left( {\frac{m}{n} = \frac{{0.348}}{{3.18 \times {{10}^{ - 3}}}} = } \right)109{\text{ }}({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
(i) (dilute aqueous) NaOH/sodium hydroxide / KOH/potassium hydroxide;
Do not accept hydroxide/OH–.
(ii) 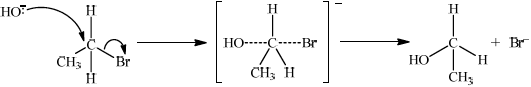
curly arrow going from lone pair/negative charge on O in HO– to C;
Do not allow curly arrow originating on H in HO–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented.
bonds broken:
1(C=C) \( + 1\) (H–Br) / \((612 + 366 = )978{\text{ (kJ)}}\);
Accept 2630 (kJ).
bonds formed:
1(C–C) \( + 1\) (C–H) \( + 1\) (C–Br) / \((1 \times 347 + 1 \times 413 + 1 \times 290 = )1050{\text{ (kJ)}}\);
Accept 2702 (kJ).
\(\Delta H = - 72{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Award [2 max] for +72 (kJ mol−1).
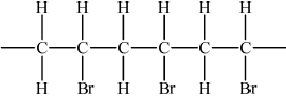 ;
;
Extension bonds required.
Ignore brackets and n.
Examiners report
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.

