| Date | May 2015 | Marks available | 3 | Reference code | 15M.2.hl.TZ2.10 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Identify and Outline | Question number | 10 | Adapted from | N/A |
Question
Some reactions of but-2-ene are given below.
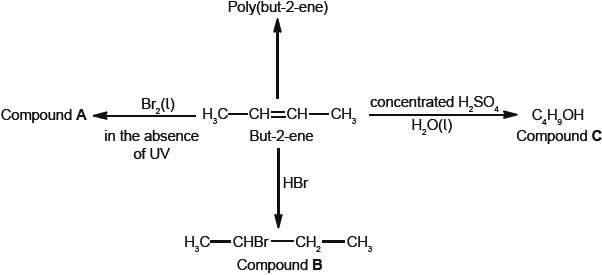
But-2-ene can exist as two geometrical isomers. Cis-trans is a form of stereoisomerism.
Deduce the full structural formula of compound A.
Apply IUPAC rules to name compound A.
Describe the colour change observed when excess but-2-ene reacts with bromine to form compound A.
(i) Outline two reasons why the polymerization of alkenes is of economic importance.
(ii) Identify the structure of the repeating unit of poly(but-2-ene).
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can also be formed by reacting compound B, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), with aqueous potassium hydroxide. This reaction proceeds by both \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms. Explain the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism, using curly arrows to represent the movement of electron pairs.
Explain why the hydroxide ion is a better nucleophile than water.
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can be oxidized by acidified potassium dichromate(VI) to form compound F.
(i) State the name of the functional group present in compound F.
(ii) Deduce the structural formula of an alcohol which is a structural isomer of compound C and cannot be oxidized by acidified potassium dichromate(VI).
Explain why but-2-ene is more volatile than compound C.
Deduce the equation for the complete combustion of compound C.
Define the term stereoisomers.
State the conditions needed for a compound to show cis-trans.
Draw the structures of the two geometrical isomers of but-2-ene, clearly identifying each as cis or trans.
Markscheme
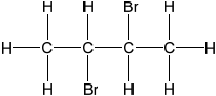 ;
;
Accept bromine atoms cis to each other.
2,3-dibromobutane;
Do not penalize the incorrect use of spaces, comma or hyphen.
red/brown/orange/yellow to colourless/decolourized;
Do not accept clear.
Do not accept just “decolorized”.
(i) (synthesis of) plastics/polymers/organic materials not naturally available / synthetic materials;
wide range of uses/physical properties / versatile;
large industry / many tons of plastics consumed by society / OWTTE;
Do not accept “useful” for M2.
Award [1 max] if specific addition polymer and its use is given.
Penalize reference to condensation polymers once only.
(ii) 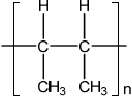 ;
;
Ignore n.
Brackets are not required for the mark, but continuation bonds are.
Do not penalize if methyl groups are trans to each other.
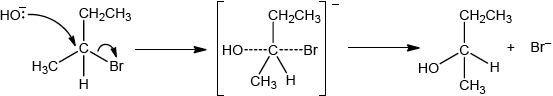
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not accept curly arrow originating on H in \(H{O^ - }\).
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 2-bromobutane or in the transition state.
Accept if arrow goes from C–Br bond to/or beyond Br.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH----C bond is represented.
formation of organic product \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHOHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\) and \({\text{KBr/B}}{{\text{r}}^ - }\);
\({\text{O}}{{\text{H}}^ - }\) has a negative charge/higher electron density;
stronger attraction to the carbon atom with the partial positive charge / OWTTE;
Do not accept just stronger attraction.
Reference to carbon atom needed for M2.
(i) carbonyl;
Accept ketone.
(ii) 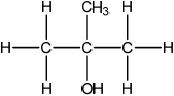 ;
;
Accept condensed or full structural formula.
hydrogen bonding in compound C;
dipole-dipole forces in C / C is more polar;
C has greater molar mass/more dispersion/London/instantaneous induced dipole-induced dipole forces/van der Waal forces;
Accept converse argument.
Award [1 max] for stronger intermolecular forces.
\({{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} + {\text{6}}{{\text{O}}_2}{\text{(g)}} \to {\text{4C}}{{\text{O}}_2}{\text{(g)}} + {\text{5}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\);
Ignore state symbols.
compounds with the same structural formula and different arrangement in space/3D structures;
Accept molecular formula instead of structural formula.
Do not accept “similar” instead of “same”.
restricted rotation around a (double) bond;
carbon atoms of the C=C/carbon-carbon double bond (in alkene)/carbon atoms of the C–C/carbon-carbon single bond (in cycloalkane) must have two different atoms/groups of atoms / OWTTE;
Do not accept “functional groups” for “groups of atoms” in M2.

Award [1 max] if cis and trans isomers are correctly drawn and identified for alkene other than but-2-ene.
Award [1 max] if student draws and labels one structure correctly but not the other.
Examiners report
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.
Most candidates were able to give the full structural formula but marks were lost by some as they gave the condensed formula rather than the full structural formula as demanded by the question. Most were able to apply IUPAC rules and name A but some omitted the “di” from dibromobutane. The colour change observed when but-2-ene reacts with bromine was well known, but knowledge of the economic importance of the polymerisation of alkenes was limited with many candidates restricting their answers to identifying specific plastics such a polythene. Many responses included incorrect references to nylon and margarine. Most candidates were able to identify the repeating unit of poly(but-2-ene). The explanation of the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism was more successful than in previous sessions although a common error was a curly arrow originating from the hydrogen atom in the hydroxide ion rather than the oxygen. Most candidates were able to explain the higher reactivity of the hydroxide ion compared to the water molecule in terms of charge but only a minority referred to the attraction between the nucleophile and low electron density of the carbon atom. The naming of 2-methylbutanenitrile was generally well done although small errors were accepted and the reagents needed for the hydrogenation of 2-methylbutanenitrile were also generally known. A number of candidates omitted the branching methyl group in the amide formed with ethanoic acid and confused aldehydes with ketone and only a small minority referred to the carbonyl group. Most candidates identified only hydrogen bonds in compound C and did not refer to the dipole-dipole forces or van der Waals’ forces also present or explicitly compare the relative strength of the different intermolecular forces in the two molecules. Some incorrectly referred to covalent bonding in their explanation. The equation for the complete combustion of compound C was generally well known. The term stereoisomer was well understood but many candidates did not refer to the restricted rotation around a double bond. Most candidates were able draw the structures of cis and trans but-2-ene.

