| Date | May 2013 | Marks available | 1 | Reference code | 13M.2.sl.TZ2.7 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Identify | Question number | 7 | Adapted from | N/A |
Question
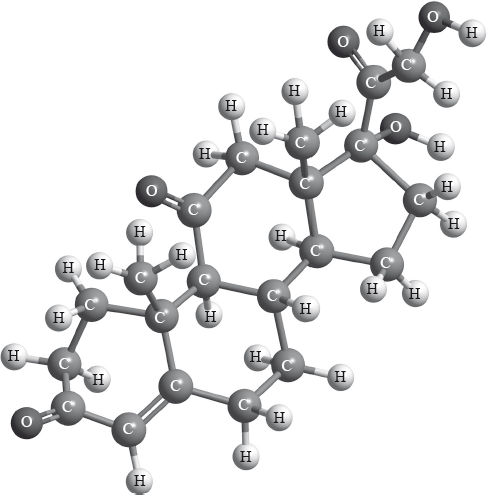
Cortisone is a therapeutic drug whose three-dimensional structure is represented below.
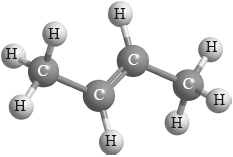
P
Menthol can be used in cough medicines. The compound contains 76.84% C, 12.92% H and 10.24% O by mass.
Identify the names of two functional groups present in cortisone.
1.
2.
Draw a circle around each of these two functional groups in the structure above and label them 1 and 2 as identified in (a) (i).
Describe what is meant by the term structural isomers.
Apply IUPAC rules to state the name of P.
X is a straight-chain structural isomer of P. Draw the structure of X.
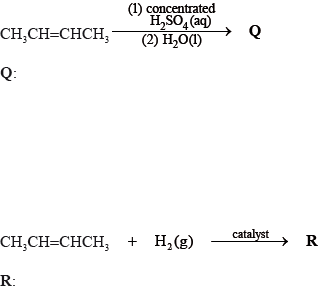
Identify a suitable catalyst used in the reaction to form R.
P, CH3CH=CHCH3, reacts with HBr to form CH3CHBrCH2CH3. Suggest one suitable mechanism for the reaction of CH3CHBrCH2CH3 with aqueous sodium hydroxide, using curly arrows to represent the movement of electron pairs.
State the structural formula of the organic product formed, S, when Q is heated under reflux with acidified potassium dichromate(VI).
Apply IUPAC rules to state the name of this product, S.
P can undergo a polymerization reaction. Draw two repeating units of the resulting polymer.
Determine its empirical formula.
Determine its molecular formula given that its molar mass is \(M = 156.30{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Markscheme
alkene;
alcohol;
Allow hydroxyl (group) but not hydroxide.
ketone;
Accept carbonyl.
correctly drawn circle around each of the two functional groups and labelled
1 and 2;
Mark can be scored for (ii) without labels (1 and 2) only if no answer is given in (i).
Apply ECF from (incorrect) functional groups in (i).
compounds with same molecular formula but different arrangements of atoms;
Allow compounds with same molecular formula but different structural formulas.
but-2-ene;
Allow 2-butene.
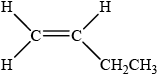 ;
;
Q: CH3CH(OH)CH2CH ;
R: CH3CH2CH2CH3;
Condensed or full structural formulas may be given.
platinum / palladium / nickel;
Allow Pt / Pd / Ni.
Since secondary bromoalkane could be either SN1 and SN2 so allow SN1 or SN2 for M1 –M4.
SN1:
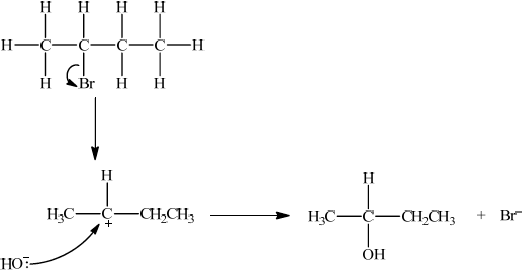
curly arrow showing Br leaving;
Do not allow arrow originating from C to C–Br bond.
representation of secondary carbocation;
curly arrow going from lone pair/negative charge on O in HO– to C+;
Do not allow arrow originating on H in OH–.
formation of CH3CH(OH)CH2CH3 and Br–;
Allow formation of NaBr instead of Br–.
OR
SN2:
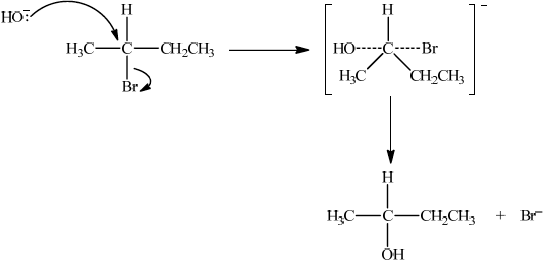
curly arrow going from lone pair/negative charge on O in HO– to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 2-bromobutane or in the transition state.
Do not allow arrow originating from C to C – Br bond.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented.
formation of CH3CH(OH)CH2CH3 and Br–;
Allow formation of NaBr instead of Br–.
H3CCOCH2CH3;
Condensed or full structural formula may be given.
Apply ECF from (c)(iii).
butan-2-one;
Allow 2-butanone or butanone.
representation of polymer showing two repeating units;
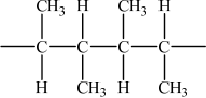
Brackets not necessary but continuation bonds must be given.
No penalty if methyl groups given on same side.
\({n_C}:\left( {\frac{{76.84}}{{12.01}}} \right) = 6.398{\text{ mol}}\) and \({n_H}:\left( {\frac{{12.92}}{{1.01}}} \right) = 12.79{\text{ mol}}\) and
\({n_O}:\left( {\frac{{10.24}}{{16.00}}} \right) = 0.6400{\text{ mol}}\);
Allow integer values for atomic masses.
dividing across by lowest number to give integer values;
\({{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
Award [3] for correct final answer.
(\(M{\text{(}}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O)}} = {\text{156.30 (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\), therefore empirical formula = molecular
formula =) \({{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
Examiners report
Question 7 was answered by relatively few candidates, but those who chose this question were usually well-prepared. In a) (i) and (ii) most candidates correctly identified two functional groups in cortisone, but some incorrectly named the ketone group as an aldehyde.
Question 7 was answered by relatively few candidates, but those who chose this question were usually well-prepared. In a) (i) and (ii) most candidates correctly identified two functional groups in cortisone, but some incorrectly named the ketone group as an aldehyde.
In b) the definition of isomers was reasonably well answered.
Most correctly named but-2-ene in c) (i). Some mistakenly said butene which was insufficient.
In c) (ii) most candidates drew the structure of but-1-ene although some drew the original compound.
In c) (iii) several candidates identified the product as butan-1-ol rather than butan-2-ol.
Nearly all identified butane as the second compound and correctly identified a suitable catalyst for this reaction in (c) (iv).
The mechanism required in c) (v) was either SN1 or SN2. Several candidates produced very clear, correct mechanisms. A few lost marks for incorrectly having a curly arrow from H instead of O in the nucleophile, or for neglecting to show the curly arrow showing Br leaving, or for omitting the negative charge on the transition state in SN2.
In c) (vi) some candidates thought that an aldehyde formed from oxidation of an alcohol under reflux. Error carried forward was applied if candidates had given butan-1-ol as the product in c) (iii) and then drew and named butanoic acid here.
Drawing two repeating units of the polymer made from but-2-ene caused many problems in c) (viii).
Parts d) (i) and (ii) were extremely well answered with most candidates determining the empirical and molecular formulas correctly.
Parts d) (i) and (ii) were extremely well answered with most candidates determining the empirical and molecular formulas correctly.

