DP Chemistry Questionbank

B.4 Carbohydrates
| Path: |
Description
[N/A]Directly related questions
-
16N.3.sl.TZ0.9b:
The structures of two molecules, X and Y, are shown below.
(i) Justify why both these molecules are carbohydrates.
(ii) Distinguish between these molecules in terms of their functional groups.
-
16N.3.sl.TZ0.9c:
Amylose is an unbranched polysaccharide composed of repeating units of glucose.
(i) Draw the structure of the repeating unit of amylose. Use section 34 of the data booklet.
(ii) Amylose is a major component of starch. Corn starch can be used to make replacements for plastics derived from oil, especially for packaging. Discuss one potential advantage and one disadvantage of this use of starch.
-
17M.3.sl.TZ1.12a.ii:
Using the partial structure given, complete the structural formula of the molecule formed from the condensation of two cyclic -glucose molecules.
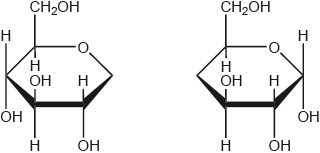
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ1.12a.i:
Deduce the straight chain structure of ribose from its ring structure drawn in section 34 of the data booklet.
-
17M.3.sl.TZ1.12b:
Constructing models that allow visualizations of the stereochemistry of carbohydrates is essential to understand their structural roles in cells.
Describe how Haworth projections help focus on the position of attached groups.
-
17M.3.sl.TZ1.11a:
List the building blocks of triglycerides and carbohydrates.
-
17M.3.sl.TZ2.10a:
Identify the functional groups which are present in only one structure of glucose.
-
17M.3.sl.TZ2.10b:
Sucrose is a disaccharide formed from -glucose and β-fructose.
Deduce the structural formula of sucrose.
-
20N.3.sl.TZ0.7b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
-
20N.3.sl.TZ0.7b(ii):
The diverse functions of biological molecules depend on their structure and shape.
Sucrose is a disaccharide formed in the reaction of glucose with fructose.
Identify the reaction type and the newly formed functional group that joins the monosaccharide units in the product.
-
20N.3.hl.TZ0.8b(i):
The diverse functions of biological molecules depend on their structure and shape.
Deduce the straight chain structure of deoxyribose from its ring structure drawn in section 34 of the data booklet.
- 17N.3.sl.TZ0.9b: Draw the structure of galactose on the skeleton provided.
-
18M.3.sl.TZ1.8b:
A person with diabetes suffering very low blood sugar (hypoglycaemia) may be advised to consume glucose immediately and then eat a small amount of starchy food such as a sandwich. Explain this advice in terms of the properties of glucose and starch.
-
18M.3.sl.TZ1.8a:
State the specific type of linkage formed between α-glucose fragments in both maltose and amylose.
-
18M.3.sl.TZ2.6e:
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
- 18N.3.hl.TZ0.10b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.sl.TZ0.8a: Name the type of link between the two monosaccharide residues.
- 18N.3.sl.TZ0.8b: Outline how the two monomer structures, galactose and glucose, differ.
- 18N.3.hl.TZ0.10a: Name the type of link between the two monosaccharide residues.
-
19M.3.hl.TZ1.8c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.hl.TZ1.8a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.hl.TZ2.12a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.hl.TZ2.12c:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
-
19M.3.sl.TZ1.7c:
Calculate the energy released, in kJ g−1, when 3.49 g of starch are completely combusted in a calorimeter, increasing the temperature of 975 g of water from 21.0 °C to 36.0 °C. Use section 1 of the data booklet.
-
19M.3.sl.TZ1.7a:
Draw the structure of the repeating unit of starch and state the type of linkage formed between these units.
Type of linkage:
-
19M.3.sl.TZ2.8a:
State the name of the functional group forming part of the ring structure of each monosaccharide unit.
-
19M.3.sl.TZ2.8b:
Sketch the cyclic structures of the two monosaccharides which combine to form sucrose.
- 19N.3.sl.TZ0.10c: Explain why maltose, C12H22O11, is soluble in water.
- 19N.3.hl.TZ0.15c: Explain why maltose, C12H22O11, is soluble in water.
