| Date | November 2009 | Marks available | 4 | Reference code | 09N.2.sl.TZ0.7 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Draw | Question number | 7 | Adapted from | N/A |
Question
Halogenoalkanes can undergo substitution reactions with potassium hydroxide solution.
State an equation for the reaction of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Cl}}\) with KOH.
Draw four structural isomers of molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\) which contain the –OH group.
On reaction with acidified potassium dichromate(VII), two of the isomers are oxidised in two steps to produce different products. Draw the structural formula of the two products formed from one of the isomers.
A third isomer is oxidized in one step. Draw the structural formula of the organic product formed.
State the colour change that takes place in these oxidation reactions.
Identify the isomer which resists oxidation by acidified potassium dichromate(VI).
Markscheme
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Cl}} + {\text{KOH}} \to {{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}} + {\text{KCl}}\);
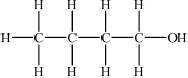 ;
;
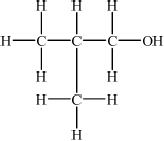 ;
;
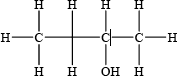 ;
;
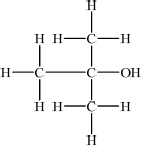 ;
;
Penalise missing H atoms once only.
Accept correct condensed structural formulas.
CH3–CH2–CH2–CHO / (CH3)2CHCHO ;
CH3–CH2–CH2–COOH / (CH3)2CHCOOH;
CH3–CH2–CO–CH3;
orange to green;
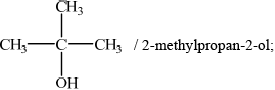
Examiners report
This was the least popular optional question but many who chose it did very well. In (a), most candidates could write the equation in (i).
Part (b) was better answered, although common errors were drawing of identical isomers in different ways in (i) and the inclusion of an extra hydrogen atom on the carbonyl group of the ketone in (iii).
Part (b) was better answered, although common errors were drawing of identical isomers in different ways in (i) and the inclusion of an extra hydrogen atom on the carbonyl group of the ketone in (iii).
Part (b) was better answered, although common errors were drawing of identical isomers in different ways in (i) and the inclusion of an extra hydrogen atom on the carbonyl group of the ketone in (iii).
Part (b) was better answered, although common errors were drawing of identical isomers in different ways in (i) and the inclusion of an extra hydrogen atom on the carbonyl group of the ketone in (iii).
Part (b) was better answered, although common errors were drawing of identical isomers in different ways in (i) and the inclusion of an extra hydrogen atom on the carbonyl group of the ketone in (iii).

