| Date | May 2014 | Marks available | 2 | Reference code | 14M.2.sl.TZ2.3 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | State | Question number | 3 | Adapted from | N/A |
Question
Hydrocarbons, such as nonane, \({{\text{C}}_{\text{9}}}{{\text{H}}_{{\text{20}}}}\), are essential as fuels and as raw materials.
Propene, which can be obtained from nonane, can be polymerized.
State a balanced equation for the complete combustion of nonane.
Combustion also often forms carbon and carbon monoxide. Outline what reaction conditions result in these being produced.
(i) State the type of polymerization that occurs.
(ii) Draw the structure of a segment of the polymer containing six carbon atoms.
Markscheme
\({{\text{C}}_9}{{\text{H}}_{20}}{\text{(l)}} + {\text{14}}{{\text{O}}_2}{\text{(g)}} \to {\text{9C}}{{\text{O}}_2}{\text{(g)}} + {\text{10}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
Do not penalize if heat given on RHS of eqn.
correct coefficients;
Ignore state symbols.
No ECF if reactants and products incorrect.
insufficient oxygen present / OWTTE;
Allow “air” instead of “oxygen”.
Do not accept “incomplete combustion”.
(i) addition (polymerization);
(ii) 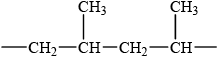 ;
;
Methyl groups must be on alternate carbons but accept other orientations.
Extension bonds required for the mark.
Allow mark if three repeating units (6 C–atoms in chain) given.
Examiners report
The range of marks students gained for this question ranged very widely, and responses were mixed. Many were able to give a balanced equation for the complete combustion of nonane although some gave hydrogen as a product and did not answer the question asked in (b) and instead referred to “incomplete combustion” as a condition. Addition polymerisation was unfamiliar to a surprising number of candidates and only the strongest candidates were able to give the structure of polypropene.
The range of marks students gained for this question ranged very widely, and responses were mixed. Many were able to give a balanced equation for the complete combustion of nonane although some gave hydrogen as a product and did not answer the question asked in (b) and instead referred to “incomplete combustion” as a condition. Addition polymerisation was unfamiliar to a surprising number of candidates and only the strongest candidates were able to give the structure of polypropene.
The range of marks students gained for this question ranged very widely, and responses were mixed. Many were able to give a balanced equation for the complete combustion of nonane although some gave hydrogen as a product and did not answer the question asked in (b) and instead referred to “incomplete combustion” as a condition. Addition polymerisation was unfamiliar to a surprising number of candidates and only the strongest candidates were able to give the structure of polypropene.

