| Date | November 2014 | Marks available | 2 | Reference code | 14N.2.sl.TZ0.7 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Define and Identify | Question number | 7 | Adapted from | N/A |
Question
Consider the following list of organic compounds.
Compound 1: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
Compound 2: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
Compound 3: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Compound 4: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = -57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Apply IUPAC rules to state the name of compound 1.
(i) Define the term structural isomers.
(ii) Identify the two compounds in the list that are structural isomers of each other.
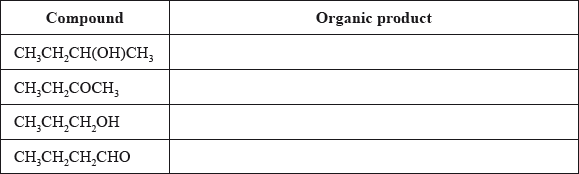
Determine the organic product formed when each of the compounds is heated under reflux with excess acidified potassium dichromate(VI). If no reaction occurs write NO REACTION in the table.
Explain the mechanism for the substitution reaction of bromoethane with sodium hydroxide. Use curly arrows to represent the movement of electron pairs.
(i) Define the term standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Markscheme
butan-2-ol/2-butanol;
(i) same molecular formula but differ in arrangement of their atoms;
Allow “different structures/structural formulas” instead of “different arrangement of atoms”.
(ii) (compounds) 2 and 4 / butanone and butanal;
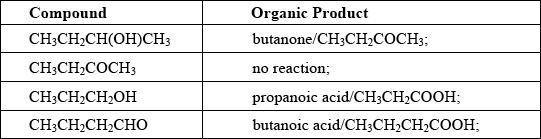
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in HO–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented, but penalise wrong bonding once only.
formation of organic product \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}\) and \({\text{B}}{{\text{r}}^ - }\);
Accept “NaBr / Na+ and Br–” as product.
If candidate writes an SN1 mechanism then deduct 1 mark for this, so that it is marked out of [3 max].
(i) heat transferred/absorbed/released/enthalpy/potential energy change when 1 mol/molar amounts of reactant(s) react (to form products) / OWTTE;
under standard conditions / at a pressure 100 kPa/101.3 kPa/1 atm and temperature 298 K/25 °C;
Award [2] for difference between standard enthalpies of products and standard enthalpies of reactants / \({H^\Theta }\) (products) – \({H^\Theta }\) (reactants).
Award [2] for difference between standard enthalpies of formation of products and standard enthalpies of formation of reactants / \(\Sigma \Delta H_f^\Theta \) (products) – \(\Sigma \Delta H_f^\Theta \) (reactants).
(ii) \((1.00 \times 0.0500 = ){\text{ }}0.0500{\text{ (mol)}}\);
\((0.0500 \times 57.9 = ){\text{ }}2.90{\text{ (kJ)}}\);
Ignore any negative sign.
Award [2] for correct final answer.
Award [1 max] for 2900 J.
(iii) \(\left( {\frac{{2.50}}{{40.00}} = } \right){\text{ }}0.0625{\text{ (mol NaOH)}}\);
\(0.0500 \times 4.18 \times 13.3 = 2.78{\text{ (kJ)}}/50.0 \times 4.18 \times 13.3 = 2780{\text{ (J)}}\);
\(\left( {\frac{{2.78}}{{0.0625}}} \right) = - 44.5{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Negative sign is necessary for M3.
Award M2 and M3 if 52.5 g is used to obtain an enthalpy change of –46.7.
(iv) –44.5 – 57.9 / correct Hess’s Law cycle (as below) / correct manipulation of equations;
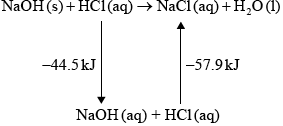
–102.4 (kJ);
Award [2] for correct final answer.
Examiners report
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.
Most students scored well on naming the required compound from its formula in Part (a), likewise defining structural isomers and recognising compounds related in the way, required in Part (b), were rarely a challenge. In Part (c) students could usually identify whether compounds underwent oxidation and the products formed, with the most common mistake being to fail to notice that there was excess dichromate(VI) in the case of the primary alcohol. The mechanism required in Part (d) seemed to be known to many, though many candidates continue to lose marks through a lack of precision about the start and finish points of curly arrows. Many students gained at least one mark for the definition standard enthalpy change in the first section of Part (e), though few displayed the precision required for both marks. In the second section quite a few tried to solve the enthalpy problem by calorimetry rather than using the enthalpy of reaction that had been given. Generally speaking the next section, that did require calorimetry, was better done though the calculation of the amount of reagent and using the mass of liquid rather than solid for the heat evolved proved a challenge for some. Many candidates correctly combined their results, sometimes invoking Hess’ Law, in the final section, though many candidates benefited from the application of ECF.

