| Date | November 2014 | Marks available | 2 | Reference code | 14N.2.hl.TZ0.9 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Define and Identify | Question number | 9 | Adapted from | N/A |
Question
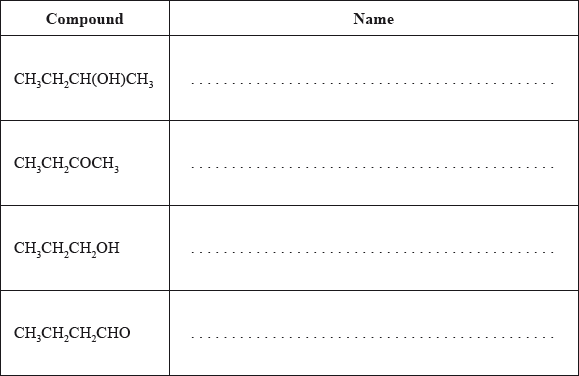
Consider the following list of organic compounds.
Compound 1: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
Compound 2: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
Compound 3: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Compound 4: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
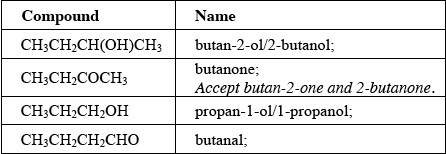
Apply IUPAC rules to state the names of the four compounds.
(i) Define the term structural isomers.
(ii) Identify the two compounds in the list that are structural isomers of each other.
(i) Determine the organic product formed when each of the compounds is heated under reflux with excess acidified potassium dichromate(VI). If no reaction occurs write NO REACTION in the table.
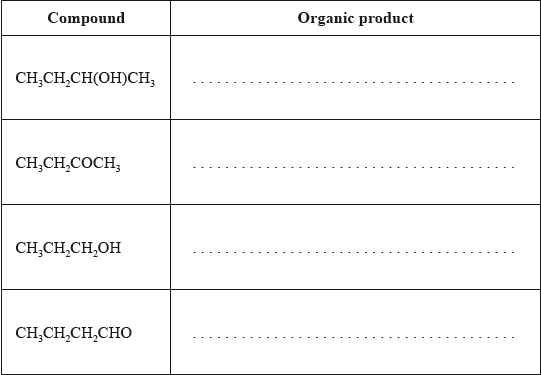
(ii) Describe the colour change during the reactions that occur in part (i).
(i) Pentanoic acid reacts with ethanol. State the structural formula of the organic product and the name of the functional group it contains.
(ii) State the type of reaction in part (i).
Describe what is meant by a weak Brønsted-Lowry base, including an equation for the reaction of ammonia with water.
Markscheme
(i) same molecular formula but differ in arrangement of their atoms;
Allow “different structures/structural formulas” instead of “different arrangement of atoms”.
(ii) (compounds) 2 and 4 / butanone and butanal;
(i) 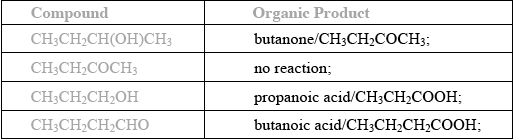
(ii) orange to green;
(i) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\);
ester;
(ii) condensation / addition-elimination;
Accept esterification.
a base is a proton acceptor;
weak means it is only partially ionized/dissociated (in solution/water);
\({\text{N}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{NH}}_4^ + + {\text{O}}{{\text{H}}^ - }{\text{ }}\);
Reversible arrow is required for M3.
Examiners report
The naming of the organic compounds was generally well answered, though quite frequently candidates benefited from the decision not to penalise the unnecessary “2” in “butan-2-one”. Propanol was a frequent incorrect answer.
(i) Generally well answered but there were some answers that reflected a poor understanding of structural isomerism.
(ii) Well answered.
(i) Generally well answered. But some candidates gave propanal as the product for propan-1-ol, and other candidates were confused about the products of the oxidation of alcohols.
(ii) Most candidates knew the colour change of the dichromate solution during the reaction.
(i) Most candidates identified and drew the ester correctly.
(ii) Very well answered.
About half of the candidates gained full marks. Many candidates omitted the reversible arrow. Some candidates only answered part of the question.

