| Date | November 2010 | Marks available | 4 | Reference code | 10N.2.sl.TZ0.5 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Deduce and Identify | Question number | 5 | Adapted from | N/A |
Question
Consider the following sequence of reactions.
\[{\text{RC}}{{\text{H}}_3}\xrightarrow{{reaction 1}}{\text{RC}}{{\text{H}}_2}{\text{Br}}\xrightarrow{{reaction 2}}{\text{RC}}{{\text{H}}_2}{\text{OH}}\xrightarrow{{reaction 3}}{\text{RCOOH}}\]
\({\text{RC}}{{\text{H}}_{\text{3}}}\) is an unknown alkane in which R represents an alkyl group.
The mechanism in reaction 2 is described as SN2.
Propan-1-ol has two structural isomers.
The alkane contains 81.7% by mass of carbon. Determine its empirical formula, showing your working.
Equal volumes of carbon dioxide and the unknown alkane are found to have the same mass, measured to an accuracy of two significant figures, at the same temperature and pressure. Deduce the molecular formula of the alkane.
(i) State the reagent and conditions needed for reaction 1.
(ii) State the reagent(s) and conditions needed for reaction 3.
Reaction 1 involves a free-radical mechanism. Describe the stepwise mechanism, by giving equations to represent the initiation, propagation and termination steps.
(i) State the meaning of each of the symbols in SN2.
(ii) Explain the mechanism of this reaction using curly arrows to show the movement of electron pairs, and draw the structure of the transition state.
(i) Deduce the structural formula of each isomer.
(ii) Identify the isomer from part (f) (i) which has the higher boiling point and explain your choice. Refer to both isomers in your explanation.
Markscheme
\({{\text{n}}_{\text{C}}} = \frac{{81.7}}{{12.01}} = 6.80\) and \({{\text{n}}_{\text{H}}} = \frac{{18.3}}{{1.01}} = 18.1\);
ratio of 1: 2.67 /1: 2.7;
\({{\text{C}}_3}{{\text{H}}_8}\);
No penalty for using 12 and 1.
\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}\);
(i) \({\text{B}}{{\text{r}}_2}\) /bromine;
UV/ultraviolet light;
Accept hf/hv/sunlight.
(ii) \({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }\) / \({\text{MnO}}_4^ - \) and acidified/\({{\text{H}}^ + }\) /\({{\text{H}}_3}{{\text{O}}^ + }\);
Accept names.
heat / reflux;
initiation:
\({\text{B}}{{\text{r}}_2} \to 2{\text{Br}} \bullet \);
propagation:
\({\text{Br}} \bullet + {\text{RC}}{{\text{H}}_3} \to {\text{HBr}} + {\text{RC}}{{\text{H}}_2} \bullet \);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{RC}}{{\text{H}}_2}{\text{Br}} + {\text{Br}} \bullet \);
termination:
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{Br}} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{Br}}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{RC}}{{\text{H}}_2} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{R}}\);
Award [1] for any termination step.
Accept radical with or without \( \bullet \) throughout.
Do not penalise the use of an incorrect alkane in the mechanism.
(i) substitution and nucleophilic and bimolecular/two species in rate-determining step;
Allow second order in place of bimolecular.
(ii) 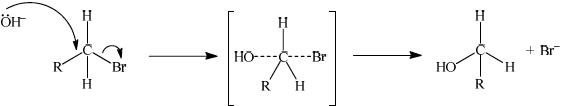
curly arrow going from lone pair/negative charge on O in OH– to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180o to each other.
Do not award M3 if OH----C bond is represented unless already penalised in M1.
Do not penalise the use of an incorrect alkyl chain in the mechanism.
(i) \({\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
\({\text{C}}{{\text{H}}_3}{\text{CHOHC}}{{\text{H}}_3}\);
Allow more detailed structural formulas.
(ii) \({\text{C}}{{\text{H}}_3}{\text{CHOHC}}{{\text{H}}_3}\) has higher boiling point due to hydrogen bonding;
\({\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) has lower boiling point due to Van der Waals’/London/dispersion/dipole-dipole forces;
Hydrogen bonds in \({\text{C}}{{\text{H}}_3}{\text{CHOHC}}{{\text{H}}_3}\) are stronger;
Allow ecf if wrong structures suggested.
Examiners report
This was the least popular question in Section B but there was a generally pleasing level of performance. Most candidates scored at least 2 out of 3 marks for calculating the empirical formula. Several candidates correctly worked out the ratio but then rounded 2.7 to 3 to give an incorrect empirical formula of \({\text{C}}{{\text{H}}_{\text{3}}}\) instead of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}\).
Many did manage to calculate a correct molecular formula even though their empirical formula was incorrect.
Free radical substitution was well known, however, there was some confusion about whether the reagent was supposed to be Br2(g), Br2(aq) or Br2 in CCl4. Most stated that UV was required.
In 5(d) most candidates scored at least 3 marks out of 4. A few used Cl2 instead of Br2.
Most knew the meaning of the symbols SN2, however, a few did not correctly state the meaning of the 2. The mechanism caused some problems and some of the common errors here were drawing the curly arrow from the H; forgetting to include any curly arrow to show Br leaving; writing the partial bond from the nucleophile as OH---C; or missing the negative charge from the transition state. Unfortunately, most candidates had a combination of these errors. Also, in most cases the partial bonds were drawn at angles less than 180 degrees which, although not penalised, is totally incorrect as attack by the nucleophile must be on the opposite side to the halogen leaving.
Part (f) proved to be very confusing for many candidates. The structural isomers of propan-1-ol were commonly drawn as propan-1-ol and propan-2-ol, which then caused enormous difficulties in 5(f)(ii) when they had to identify the isomer with the higher boiling point.
Those who were relying on ECF marks here often predicted the wrong isomer or found it very difficult to explain their prediction. The few candidates who drew the isomers correctly as an ether and an alcohol were generally able to score full marks by predicting and explaining the different boiling points.

