| Date | May 2012 | Marks available | 2 | Reference code | 12M.3.sl.TZ1.G1 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | G1 | Adapted from | N/A |
Question
The structure of benzene originally described by August Kekulé is shown below.
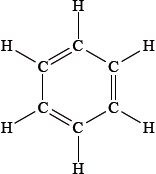
Explain, giving two different pieces of evidence, why this is not a valid structure for the bonding in benzene.
Markscheme
all (C–C) bond lengths equal / C–C bond lengths intermediate between C–C and C=C;
benzene normally undergoes substitution not addition;
thermochemically more stable than predicted / produces less heat when hydrogenated/combusted than predicted;
Examiners report
This part was generally well answered by many candidates.
Syllabus sections
Show 183 related questions
- 14N.1.sl.TZ0.27: Which structural formula represents a secondary halogenoalkane? A. ...
- 14N.2.sl.TZ0.4a: State the names of two functional groups in D-fructose.
- 13N.1.hl.TZ0.35: What is the name of...
- 13M.2.hl.TZ2.8c: Clomifene, a fertility drug, whose three-dimensional structure is represented below, also has...
- 13M.3.hl.TZ2.D3b.i: Because it contains several –OH groups and an amine group, doxycycline is slightly polar....
- 09N.2.hl.TZ0.9a.i: Draw the structural formulas of the two geometrical isomers of 1-chloro-but-2-ene.
- 09N.2.hl.TZ0.9a.ii: Explain why 1-chloro-but-2-ene shows geometrical isomerism.
- 09N.1.sl.TZ0.26: How many structural isomers exist with the formula...
- 09M.2.hl.TZ2.5f.iv: State the type of hybridization of the carbon and nitrogen atoms in...
- 09M.2.sl.TZ2.1a: State the names of the three organic functional groups in aspirin.
- 11M.2.sl.TZ1.6a: State and explain the trend of the boiling points of the first five members of the alkene...
- 11M.1.sl.TZ2.26: Which organic molecule is not a structural isomer of pentan-1-ol? A. pentan-2-ol B. ...
- 12M.1.hl.TZ2.32: What is the name of...
- 16N.3.sl.TZ0.20a: Compare and contrast the functional groups present in methadone and diamorphine (heroin),...
- 17M.2.sl.TZ1.6b: State the typical reactions that benzene and cyclohexene undergo with bromine.
- 17M.3.sl.TZ1.10b.i: Describe the difference in their structures.
- 17M.1.sl.TZ1.24: What is the order of increasing boiling point? A. C4H10 < CH3COOH < CH3CH2CHO <...
- 17N.1.sl.TZ0.28: How many structural isomers of C6H14 exist? A. 4 B. 5 C. 6 D. 7
- 259336: This is an example question for the example test. You can delete this question.
- 15M.1.sl.TZ2.26: Applying IUPAC rules, what is the name of...
- 15M.2.sl.TZ2.7e: Compound C can be oxidized by acidified potassium dichromate(VI) to form compound F. (i) ...
- 13N.1.hl.TZ0.36: Which functional groups are present in...
- 13M.2.sl.TZ1.5b: Deduce the structural formula of two isomers of the molecule above with the same functional...
- 13M.2.sl.TZ2.7b: Describe what is meant by the term structural isomers.
- 13M.2.sl.TZ2.7c.vii: Apply IUPAC rules to state the name of this product, S.
- 12N.1.sl.TZ0.27: Which compound is not an isomer of hexane? A. ...
- 12N.2.sl.TZ0.6a.ii: State the meaning of the term structural isomers.
- 10M.3.sl.TZ1.G3b: Explain how the reaction of benzene with bromine provides chemical evidence that benzene does...
- 09M.1.hl.TZ1.35: What is the IUPAC name for...
- 09M.2.sl.TZ1.2a: Calculate the value for the enthalpy of hydrogenation of ethene obtained using the average...
- 09M.1.hl.TZ2.38: What is the IUPAC name of the compound...
- 09M.1.sl.TZ2.27: Which is a tertiary halogenoalkane? A. ...
- 16N.3.sl.TZ0.10a: State the IUPAC name for leucine.
- 16N.1.sl.TZ0.24: Which alcohols are oxidized by acidified potassium dichromate(VI) solution when heated? A....
- 17M.1.sl.TZ1.26: What is the name of the compound with this molecular structure applying IUPAC rules? A. ...
- 17M.2.sl.TZ1.5c.ii: The mass and 1H\(\,\)NMR spectra of product X are shown below. Deduce, giving your reasons,...
- 17M.2.hl.TZ1.6c.ii: The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its...
- 17M.1.hl.TZ2.36: Which compounds can be reduced? I. C2H4II. CH3COOHIII. CH3CHO A. I and II...
- 17M.2.sl.TZ2.6b: Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react...
- 17M.2.hl.TZ2.7b.i: Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react...
- 17M.3.hl.TZ2.20a.iii: Suggest two absorbances, other than the absorbances due to the ring structure and C–H bonds,...
- 14M.1.sl.TZ2.29: In which pair are both compounds secondary?
- 14M.1.sl.TZ2.27: Which properties are features of a homologous series? I. Same general formula II. ...
- 14M.1.sl.TZ2.28: Which compound is an isomer of octane,...
- 14N.2.hl.TZ0.9a: Apply IUPAC rules to state the names of the four compounds.
- 13N.2.sl.TZ0.6a: Draw the structure of 2-methylbut-2-ene.
- 13M.1.sl.TZ1.25: What is the name of the following molecule applying IUPAC rules? A. ...
- 13M.2.hl.TZ2.8d.i: Draw any two other isomers of P.
- 13M.2.sl.TZ2.4b.i: Deduce the full structural formula for both compounds, showing all the bonds...
- 12N.2.hl.TZ0.6e.i: Apply IUPAC rules to name the ester, CH3COOCH2CH3(aq).
- 12N.1.sl.TZ0.26: Which statement about a homologous series is correct? A. Members of the series differ by...
- 10N.2.sl.TZ0.5f: (i) Deduce the structural formula of each isomer. (ii) Identify the isomer from part...
- 10N.3.sl.TZ0.G1: (a) Describe the structure of benzene, C6H6. (b) State two pieces of evidence that...
- 10M.2.hl.TZ1.7a: Below are four structural isomers with molecular formula...
- 09M.2.sl.TZ1.7a.v: Identify the class of alcohols that propan-2-ol belongs to and state the name of the organic...
- 09M.2.hl.TZ2.2a.ii: Based on its \({K_{\text{a}}}\) value, state and explain whether benzoic acid is a strong or...
- 11M.1.sl.TZ1.28: Which of the following are isomers of pentane? I. 2-methylpentane II. ...
- 11M.1.sl.TZ1.29: Which of the following pairs are members of the same homologous series? A. ...
- 11M.2.sl.TZ1.6b: Describe two features of a homologous series.
- 11M.2.sl.TZ1.6d: Describe a chemical test that could be used to distinguish between pent-1-ene and pentane.
- 12M.1.sl.TZ2.26: Consider the compound...
- 11N.2.hl.TZ0.6d.i: Compare the formation of sigma (\(\sigma \)) and pi (\(\pi \)) bonds between the carbon atoms...
- 11N.2.hl.TZ0.9b.i: State the IUPAC names of each of the compounds, D, E, F and G. D: E: F: G:
- 16M.2.hl.TZ0.5a: (i) State the term that is used to describe molecules that are related to each other in the...
- 17M.2.sl.TZ1.6a: Discuss the physical evidence for the structure of benzene.
- 17M.2.hl.TZ1.6c.i: One possible product, X, of the reaction of ethane with chlorine has the...
- 17M.1.sl.TZ2.27: Which compound contains a secondary carbon atom? A. CH3CH(Cl)CH(CH3)2 B. ...
- 17M.1.sl.TZ2.24: Which functional group is present in paracetamol? A. Carboxyl B. Amino C. ...
- 17M.2.hl.TZ2.2a.v: Identify one organic functional group that can react with acidified K2Cr2O7(aq).
- 17M.2.hl.TZ2.7a.ii: Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of...
- 15M.2.sl.TZ1.7b: \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) exists as three isomers. Identify the...
- 14M.3.hl.TZ2.3e: Y is an isomer of X, which contains the same functional groups. Deduce the structural formula...
- 14M.2.sl.TZ2.6a: Applying IUPAC rules, state the name of A.
- 14M.2.sl.TZ2.6g.ii: State the homologous series to which D belongs.
- 13M.2.sl.TZ1.8a.i: Outline three features of a homologous series.
- 12N.2.sl.TZ0.6a.iii: X is an isomer of C4H8 and has the structural formula shown below. Apply IUPAC rules to...
- 12N.2.sl.TZ0.6b.i: Draw the ester functional group.
- 10N.1.sl.TZ0.27: Which order is correct when the following substances are arranged in order of increasing...
- 10M.2.sl.TZ1.6b: Ethanol is part of the homologous series of alcohols. Describe two features of a homologous...
- 10M.2.sl.TZ1.6c: (i) Below are four structural isomers of alcohols with molecular formula...
- 09M.2.hl.TZ1.2a.i: State the equation for the reaction of propanoic acid with water.
- 09M.3.sl.TZ1.D1c.i: Identify the amine functional group in the morphine molecule below by drawing a ring around...
- 11M.2.sl.TZ1.6c: Below is a schematic diagram representing some reactions of ethene. The letters A–D represent...
- 11M.1.sl.TZ2.27: Which of the structures below is an aldehyde? A. ...
- 12M.2.sl.TZ2.7a: (i) State the meaning of the term isomers. (ii) Deduce the structural formulas of...
- 11N.2.hl.TZ0.9b.iv: Discuss the volatility of E compared to F.
- 11N.1.sl.TZ0.26: Which molecule contains an ester group? A. ...
- 11N.2.sl.TZ0.4b: Deduce the balanced chemical equation for the complete combustion of butan-1-ol.
- 16M.2.sl.TZ0.4b: Compound B is related to compound A. (i) State the term that is used to describe molecules...
- 16M.3.sl.TZ0.8b: (i) State the name of the functional group circled in the DHEA molecule shown below. (ii)...
- 16N.2.sl.TZ0.5a: Draw the full structural formulas of propane and propene.
- 16N.1.sl.TZ0.23: The structure of a drug used to treat symptoms of Alzheimer’s disease is shown below. Which...
- 17M.2.hl.TZ1.7c: State the reagents used to convert benzene to nitrobenzene and the formula of...
- 17M.2.sl.TZ1.5a: Ethane, C2H6, reacts with chlorine in sunlight. State the type of this reaction and the name...
- 17M.2.hl.TZ2.7a.iii: Suggest the structural formula of this compound.
- 17N.1.sl.TZ0.26: What is the name of this compound, using IUPAC rules? A. 3-methylbutan-3-ol B....
- 14M.1.hl.TZ2.39: What is the structural formula of the ester formed by reacting propanoic acid with...
- 14M.1.hl.TZ1.34: What is the IUPAC name for...
- 14M.1.sl.TZ1.26: Which statement is correct for members of the same homologous series? A. They have the...
- 14M.3.sl.TZ2.20b: State one piece of chemical evidence proving benzene does not contain alternate single and...
- 14N.2.hl.TZ0.9b: (i) Define the term structural isomers. (ii) Identify the two compounds in the...
- 13M.2.sl.TZ1.5a: Apply IUPAC rules to state the name of this molecule.
- 13M.2.sl.TZ1.8c.iii: Deduce whether C4H9Br is a primary or tertiary halogenoalkane.
- 13M.2.sl.TZ1.8c.iv: Determine the structural formula of C4H9Br.
- 13M.3.sl.TZ1.A1a: Draw the two possible structures of compound P.
- 13M.1.sl.TZ2.26: Which three compounds can be considered to be a homologous series? A. ...
- 13M.2.sl.TZ2.7a.ii: Draw a circle around each of these two functional groups in the structure above and label...
- 13M.2.sl.TZ2.7c.i: Apply IUPAC rules to state the name of P.
- 10N.1.sl.TZ0.26: Which of the following substances are structural isomers of each other? I. ...
- 10N.2.sl.TZ0.2a: State the name of the alkene shown.
- 09M.2.sl.TZ1.7a.iv: Propan-2-ol is an isomer of propan-1-ol. Draw the structure of propan-2-ol.
- 09M.2.sl.TZ1.1a: Identify the organic functional group present in both vegetable oil and biodiesel.
- 09M.1.sl.TZ1.27: What is the IUPAC name for...
- 09M.2.hl.TZ2.2a.iii: Determine the hydrogen ion concentration and the pH of a...
- 11M.3.sl.TZ2.D2b.i: State the name of the functional group circled on the structure of caffeine.
- 12M.2.sl.TZ2.5a: (i) Distinguish between the terms empirical formula and molecular formula. Empirical...
- 16M.1.sl.TZ0.24: ...
- 16M.3.hl.TZ0.26a: Identify the functional group circled in the structure of oseltamivir.
- 16N.3.sl.TZ0.9b: The structures of two molecules, X and Y, are shown below. (i) Justify why both these...
- 16N.2.sl.TZ0.1b: Determine the average oxidation state of carbon in ethene and in...
- 17M.3.sl.TZ1.15b: Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the...
- 17M.2.sl.TZ1.5c.i: One possible product, X, of the reaction of ethane with chlorine has the...
- 17M.2.hl.TZ2.7c.i: State the reagents and the name of the mechanism for the nitration of benzene.
- 17N.1.hl.TZ0.38: Which functional group is responsible for the pKb of 4.1 in this compound? A. Amido B....
- 15M.2.sl.TZ1.5c: Identify the homologous series to which ethanol belongs and state two features of a...
- 15M.2.sl.TZ2.4a: State two features of a homologous series.
- 14M.3.sl.TZ2.3e: (i) Deduce the structural formula of Y. (ii) Predict one difference between the...
- 14N.1.sl.TZ0.26: What is the name of the alkane shown in the diagram below, applying IUPAC rules? A. ...
- 14N.2.sl.TZ0.7b: (i) Define the term structural isomers. (ii) Identify the two compounds in the...
- 13N.1.sl.TZ0.26: What is the name of...
- 13M.2.hl.TZ2.3a: Identify the name of the functional group circled in the structure of carboplatin.
- 13M.2.hl.TZ2.8d.vi: Apply IUPAC rules to state the name of this product, U.
- 13M.2.hl.TZ2.8d.ii: Apply IUPAC rules to state the names of all the straight-chain isomers of compounds of...
- 10N.1.hl.TZ0.37: Which compound is an amide? A. CH3COOCH3 B. CH3CONH2 C. CH3NH2 D. ...
- 10N.2.hl.TZ0.2c: Identify the structural formula of an isomer of but-2-ene which does not decolourize bromine...
- 09N.2.hl.TZ0.9c.i: State whether this reaction is SN1 or SN2.
- 09M.2.hl.TZ1.3b: Determine the value for the enthalpy of hydrogenation of ethene using the values for the...
- 09M.1.sl.TZ1.26: Which three compounds can be considered to be a homologous series? A. ...
- 09M.2.hl.TZ2.8b.i: Ethanol to ethyl ethanoate.
- 09M.1.sl.TZ2.28: What is the IUPAC name of the following compound? A. 2-methylbutane B. ...
- 12M.3.hl.TZ2.D3d: (i) Identify the \(\beta \)-lactam ring by drawing a circle around it. (ii) Explain...
- 11N.2.sl.TZ0.4d: Based on the types of intermolecular force present, explain why butan-1-ol has a higher...
- 11N.2.sl.TZ0.7a: One example of a homologous series is the alcohols. Describe two features of a homologous...
- 11N.2.sl.TZ0.7b.ii: State the reagents and reaction conditions used to convert X to Y and X to Z. X to Y: X to Z:
- 16M.2.hl.TZ0.2c: The other monomer used in the production of polyurethane is compound Z shown below. (i)...
- 17M.3.sl.TZ1.10b.ii: Explain why the difference in their structures affects their melting points.
- 17M.2.sl.TZ1.5d: Chloroethene, C2H3Cl, can undergo polymerization. Draw a section of the polymer with three...
- 17M.2.sl.TZ2.6a: Using relevant equations, show the initiation and the propagation steps for this reaction.
- 17M.2.hl.TZ2.6b: The overall equation for monochlorination of methane is: CH4(g) + Cl2(g) → CH3Cl(g) +...
- 17M.2.hl.TZ2.9b.i: Hydrogenation of propene produces propane. Calculate the standard entropy change, ΔS θ, for...
- 17M.3.hl.TZ2.3c.iii: Subsequent steps proceed under differing conditions, forming the dendrimer polymer with the...
- 15M.2.hl.TZ2.10a.ii: Apply IUPAC rules to name compound A.
- 15M.1.sl.TZ1.27: Applying IUPAC rules, what is the name of the compound? A. ...
- 15M.2.sl.TZ2.7a.ii: Apply IUPAC rules to name compound A.
- 14N.2.sl.TZ0.4b: Deduce the empirical formula of D-fructose.
- 13M.2.sl.TZ2.3a.ii: State the name of the compound formed that is responsible for this decreased pH value.
- 13M.2.sl.TZ2.7a.i: Identify the names of two functional groups present in cortisone. 1. 2.
- 09M.1.hl.TZ1.34: Identify the functional group present in...
- 09M.2.hl.TZ2.2a.i: Calculate the \({K_{\text{a}}}\) value of benzoic acid,...
- 09M.2.sl.TZ2.6b.i: State the name of one structural isomer of pentane.
- 17M.1.sl.TZ1.25: What are the functional groups in the aspirin...
- 17M.1.sl.TZ1.27: Which molecule has a tertiary nitrogen? A. (CH3)2NH B. (C2H5)4N+I− C. ...
- 17M.1.hl.TZ2.37: In which order should the reagents be used to convert benzene into phenylamine (aniline)?
- 17M.3.sl.TZ2.10a: Identify the functional groups which are present in only one structure of glucose.
- 15M.2.hl.TZ2.10e: Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can be oxidized by...
- 14M.2.sl.TZ2.3a: State a balanced equation for the complete combustion of nonane.
- 14M.3.sl.TZ2.20a: Describe the structure of benzene.
- 14N.2.sl.TZ0.7a: Apply IUPAC rules to state the name of compound 1.
- 13M.2.hl.TZ1.9a.i: Outline three features of a homologous series.
- 13M.1.sl.TZ1.26: How many non-cyclic structural isomers exist with the molecular formula...
- 13M.1.sl.TZ2.27: What is the name of the following compound applying IUPAC rules? A. ...
- 13M.2.sl.TZ2.7c.ii: X is a straight-chain structural isomer of P. Draw the structure of X.
- 09N.2.hl.TZ0.9a.iii: Draw the structural formula of one isomer of...
- 09N.2.sl.TZ0.4a.i: Identify the boiling points for each of the isomers A, B and C and state a reason for your...
- 09N.2.sl.TZ0.7b.i: Draw four structural isomers of molecular formula...
- 10M.3.sl.TZ1.G3a: Describe two different types of physical evidence which show that benzene does not contain...
- 10M.1.sl.TZ2.27: What is the structural formula of 2,3-dibromo-3-methylhexane? A. ...
- 11N.2.sl.TZ0.4c: Determine the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\),...
- 11N.2.sl.TZ0.7b.i: The IUPAC name of X is 4-methylpentan-1-ol. State the IUPAC names of Y and Z. Y: Z:
- 16M.1.sl.TZ0.23: ...
- 16M.2.sl.TZ0.4a: (i) State the name, applying IUPAC rules, of compound A. (ii) Draw a section, showing three...
- 16M.2.hl.TZ0.2b: One important industrial use of phosgene is the production of polyurethanes. Phosgene is...
- 17M.1.hl.TZ1.35: What is the major product of the reaction between 2-methylbut-2-ene and hydrogen bromide? A....

