| Date | May 2013 | Marks available | 1 | Reference code | 13M.2.hl.TZ2.3 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Identify | Question number | 3 | Adapted from | N/A |
Question
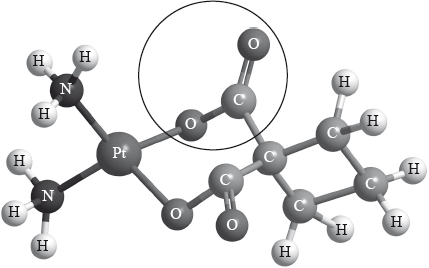
Carboplatin used in the treatment of lung cancer has the following three-dimensional structure.
Elemental platinum has electrons occupying s, p, d and f atomic orbitals.
Identify the name of the functional group circled in the structure of carboplatin.
State the type of bonding between platinum and nitrogen in carboplatin.
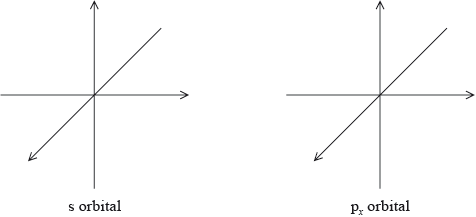
Draw the shape of an s orbital and a px orbital. Label the x, y and z axes on each diagram.
State the maximum number of orbitals in the \(n = 4\) energy level.
A number of ruthenium-based anti-cancer drugs have also been developed. State the full electron configuration of the ruthenium(II) ion, \({\text{R}}{{\text{u}}^{2 + }}\).
Iron is in the same group in the periodic table as ruthenium.
Construct the orbital diagram (using the arrow-in-box notation) for iron, showing the electrons in the \(n = 3\) and \(n = 4\) energy levels only and label each sub-level on the diagram.
Markscheme
ester;
Do not accept just carbonyl.
Allow carboxylato (ligand)/carboxylate (ligand) but not carboxyl/carboxy.
dative (covalent) / coordinate;
Do not allow just covalent or co-dative.
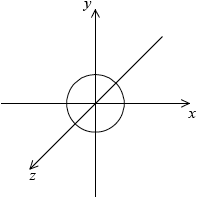
symmetrical s orbital representation;
Do not penalize if axes are not labelled for s orbital.
x, y, z can be located in any direction.
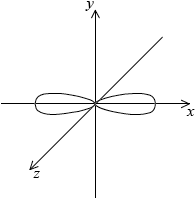
dumbbell-shaped px orbital representation with electron density located along x-axis;
x-axis must be labelled for px orbital.
Do not accept if py and pz are also drawn as question asks for orbital not sub-level.
16;
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{d}}^{\text{6}}}\);
Order of 4s and 3d levels can be interchanged.
Do not accept other notation such as subscripts.
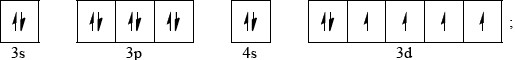
Allow full arrows instead of half-arrows in orbital diagram.
Sub-levels must be labelled for mark.
Examiners report
Many candidates identified the functional group but not the type of bond between Pt and N in carboplatin. A surprising number of candidates were unable to draw a px orbital or drew all p orbitals, or did not label the axis
Very few gave 16 as the answer.

