| Date | May 2013 | Marks available | 1 | Reference code | 13M.2.hl.TZ2.8 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Apply | Question number | 8 | Adapted from | N/A |
Question
Geometrical isomerism and optical isomerism are two sub-groups of stereoisomerism in organic chemistry.
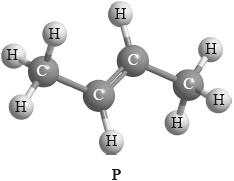
Compound P has the following three-dimensional structure. P also has geometrical isomers.
Menthol can be used in cough medicines. The compound contains C, H and O only.
Describe what is meant by the term stereoisomers.
Geometrical isomers have different physical properties and many drugs, such as doxepin (which has antidepressant properties), have geometrical isomers.
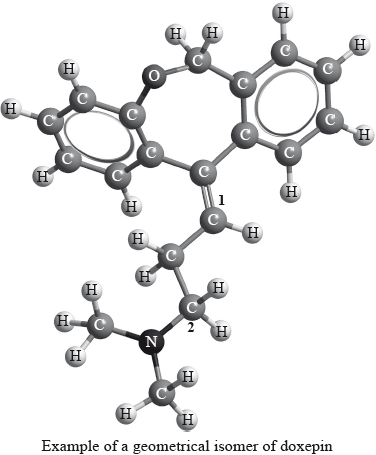
For each of the carbon atoms labelled 1 and 2 in doxepin, deduce the type of hybridization involved (sp, sp2 or sp3).
1:
2:
Clomifene, a fertility drug, whose three-dimensional structure is represented below, also has geometrical isomers.
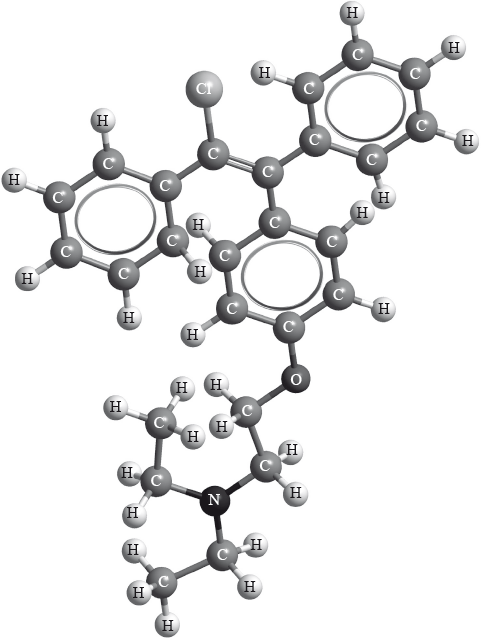
Identify the name of one functional group present in clomifene.
Draw any two other isomers of P.
Apply IUPAC rules to state the names of all the straight-chain isomers of compounds of molecular formula C4H8 (including P).
State the structural formula of the organic products, Q, R, S and T, formed in the following reactions.
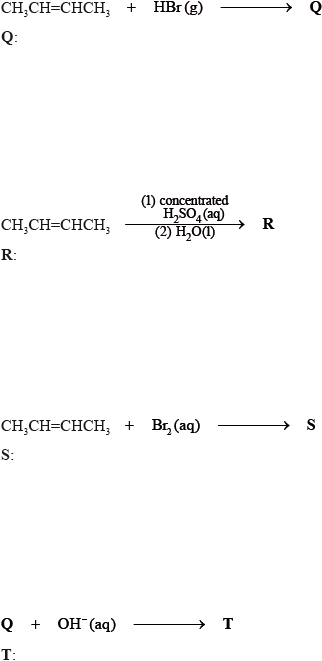
Suggest one suitable mechanism for the reaction of Q with aqueous sodium hydroxide to form T, using curly arrows to represent the movement of electron pairs.
State the structural formula of the organic product formed, U, when R is heated under reflux with acidified potassium dichromate(VI).
Apply IUPAC rules to state the name of this product, U.
When a \(6.234 \times {10^{ - 2}}{\text{ g}}\) of the compound was combusted, \(1.755 \times {10^{ - 1}}{\text{ g}}\) of carbon dioxide and \(7.187 \times {10^{ - 2}}{\text{ g}}\) of water were produced. Determine the molecular formula of the compound showing your working, given that its molar mass is \(M = 156.30{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Menthol occurs naturally and has several isomers. State the structural feature of menthol which is responsible for it having enantiomers.
State the instrument used to distinguish between each of the two enantiomers, and how they could be distinguished using this instrument.
Compare the physical and chemical properties of enantiomers.
Physical properties:
Chemical properties:
Markscheme
compounds with same structural formula but different arrangements of atoms in space;
Award [1] if correct description of geometric and optical isomers given.
1: sp2 and 2: sp3;
amine;
benzene ring;
Allow phenyl (group).
Do not allow just benzene.
alkene / chloroalkene;
chloro;
ether / phenyl ether;
Ethers not required as per guide but allow if given.
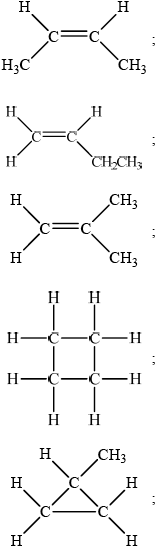
trans-but-2-ene and cis-but-2-ene;
Allow trans 2-butene and cis 2-butene.
Do not accept just 2-butene or 2-butene.
but-1-ene;
Allow 1-butene.
Q: \({\text{C}}{{\text{H}}_3}{\text{CHBrC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
R: \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
S: \({\text{C}}{{\text{H}}_3}{\text{CHBrCHBrC}}{{\text{H}}_3}\);
T: \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
Condensed or full structural formulas may be given.
Since secondary bromoalkane could be either SN1 and SN2 so allow SN1 or SN2 for M1 –M4.
SN1:
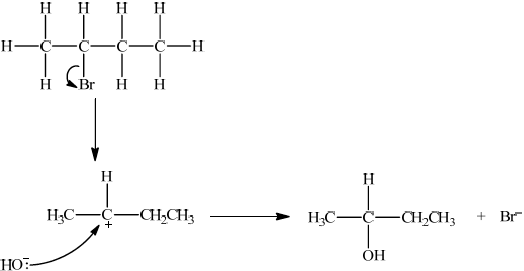
curly arrow showing Br leaving;
Do not allow arrow originating from C to C–Br bond.
representation of secondary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
Do not allow arrow originating on H in OH–.
formation of \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) and \({\text{B}}{{\text{r}}^ - }\);
Allow formation of NaBr instead of Br–.
OR
SN2:
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 2-bromobutane or in the transition state.
Do not allow arrow originating from C to C–Br bond.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented.
formation of \({\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) and \({\text{B}}{{\text{r}}^ - }\);
Allow formation of NaBr instead of Br–.
\({{\text{H}}_3}{\text{CCOC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
Condensed or full structural formula may be given.
butan-2-one;
Allow 2-butanone or butanone.
Accept butan-2-one if (v) is incorrect but also apply ECF.
\({m_{\text{C}}}:(1.755 \times {10^{ - 1}} \times 12.01)/(44.01) = 4.790 \times {10^{ - 2}}{\text{ g}}\) and
\({m_{\text{H}}}:(7.187 \times {10^{ - 2}} \times 2 \times 1.01)/(18.02) = 8.056 \times {10^{ - 3}}{\text{ g}}\);
\({m_{\text{O}}}:(6.234 \times {10^{ - 2}} - 8.056 \times {10^{ - 3}} - 4.790 \times {10^{ - 2}}) = 6.384 \times {10^{ - 3}}{\text{ g}}\);
\(({n_{\text{C}}} = 3.988 \times {10^{ - 3}}\) and \({n_{\text{H}}} = 2 \times 3.988 \times {10^{ - 3}}\) and \({n_{\text{O}}} = 3.988 \times {10^{ - 3}}\) hence empirical formula \( = {\text{) }}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
\(\left( {M{\text{(}}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O)}} = 156.30{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{), therefore molecular formula}} = } \right){\text{ }}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
OR
\({n_{{\text{C}}{{\text{O}}_2}}} = \left( {\frac{{1.755 \times {{10}^{ - 1}}}}{{44.01}}} \right) = 3.988 \times {10^{ - 3}}\) and \({n_{{{\text{H}}_2}{\text{O}}}} = \left( {\frac{{7.187 \times {{10}^{ - 1}}}}{{18.02}}} \right) = 3.988 \times {10^{ - 3}}\);
\({m_{\text{O}}}:(6.234 \times {10^{ - 2}} - 8.056 \times {10^{ - 3}} - 4.790 \times {10^{ - 2}}) = 6.384 \times {10^{ - 3}}{\text{ g}}\);
\(({n_{\text{C}}} = 3.988 \times {10^{ - 3}}\) and \({n_{\text{H}}} = 2 \times 3.988 \times {10^{ - 3}}\) and \({n_{\text{O}}} = 3.988 \times {10^{ - 3}}\) hence empirical formula \( = {\text{) }}{{\text{C}}_{{\text{10}}}}{{\text{H}}_{20}}{\text{O}}\);
\(\left( {M{\text{(}}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O)}} = 156.30{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{), therefore molecular formula }} = } \right){\text{ }}{{\text{C}}_{10}}{{\text{H}}_{20}}{\text{O}}\);
Allow alternative working to be used.
Award [3 max] for C10H20O if no working shown.
chiral (carbon/centre/atom) / (tetrahedral) carbon surrounded by four
different groups;
Accept chiral compound or chiral molecule.
polarimeter and (enantiomers) rotate plane of polarized light in (equal and) opposite directions;
Physical properties:
identical except for rotation of plane polarized light;
Accept “identical” as different optical properties assessed in (iii).
Do not accept similar.
Chemical properties:
identical unless they interact with other optically active/chiral compounds/reagents/solvents / identical with achiral compounds/reagents/solvents / OWTTE;
Allow different physiological effects/taste.
Examiners report
A reasonably popular question and often well done. In (a), some weaker candidates did not understand the idea of a stereoisomer.
(b) and (c) were well done.
(b) and (c) were well done.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis. In (ii), many did not gain marks for but-2-ene.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis.
In (d), most scored full marks though some gave cis.
(e) (i) also was very well answered compared to some recent sessions.
Perhaps too much was expected in (iii) for one mark and students either omitted polarimeter or did not refer to plane polarised light.
In (iv), few scored both marks.

