| Date | November 2014 | Marks available | 1 | Reference code | 14N.2.sl.TZ0.4 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | State | Question number | 4 | Adapted from | N/A |
Question
The open-chain structure of D-fructose is shown below.
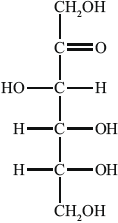
State the names of two functional groups in D-fructose.
Deduce the empirical formula of D-fructose.
Calculate the percentage composition by mass of D-fructose.
State a balanced equation for the complete combustion of D-fructose.
Markscheme
hydroxyl and carbonyl;
Accept alcohol as an alternative to hydroxyl and/or ketone as an alternative to carbonyl.
Allow hydroxy, but not hydroxide as an alternative to hydroxyl.
\({\text{C}}{{\text{H}}_2}{\text{O}}\);
C: \(\left( {\frac{{12.01}}{{30.03}} \times 100 = } \right){\text{ }}39.99/40.0\% \)
H: \(\left( {\frac{{2.02}}{{30.03}} \times 100 = } \right){\text{ }}6.73/6.7\% \)
O: \(\left( {\frac{{16.00}}{{30.03}} \times 100 = } \right){\text{ }}53.28/53.3\% \);
Award [2] if all three are correct, and [1] if two are correct.
Accept if the third value is obtained by subtracting the other two percentages from 100%.
Do not penalize if integer values of relative atomic masses are used.
\({{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_6} + {\text{6}}{{\text{O}}_2} \to {\text{6C}}{{\text{O}}_2} + {\text{6}}{{\text{H}}_2}{\text{O}}\)
correct formulas of reactants and products;
correct balancing;
M2 can only be scored if M1 correct.
Examiners report
The functional groups in fructose proved a challenge for only the weakest candidates, with mistaking the carbonyl group for “aldehyde” being the most common error. Please note that to prepare new candidates for the 2016 syllabus, the markscheme was later altered to include the correct naming of functional groups following IUPAC guidelines. Many students could also correctly convert the structural formula into an empirical formula in Part (b) and then went on to correctly determine the percentage by mass of each element in Part (c), though sometimes only with the help of ECF. Writing the correct combustion equation was difficult for only the weaker candidates.
The functional groups in fructose proved a challenge for only the weakest candidates, with mistaking the carbonyl group for “aldehyde” being the most common error. Please note that to prepare new candidates for the 2016 syllabus, the markscheme was later altered to include the correct naming of functional groups following IUPAC guidelines. Many students could also correctly convert the structural formula into an empirical formula in Part (b) and then went on to correctly determine the percentage by mass of each element in Part (c), though sometimes only with the help of ECF. Writing the correct combustion equation was difficult for only the weaker candidates.
The functional groups in fructose proved a challenge for only the weakest candidates, with mistaking the carbonyl group for “aldehyde” being the most common error. Please note that to prepare new candidates for the 2016 syllabus, the markscheme was later altered to include the correct naming of functional groups following IUPAC guidelines. Many students could also correctly convert the structural formula into an empirical formula in Part (b) and then went on to correctly determine the percentage by mass of each element in Part (c), though sometimes only with the help of ECF. Writing the correct combustion equation was difficult for only the weaker candidates.
The functional groups in fructose proved a challenge for only the weakest candidates, with mistaking the carbonyl group for “aldehyde” being the most common error. Please note that to prepare new candidates for the 2016 syllabus, the markscheme was later altered to include the correct naming of functional groups following IUPAC guidelines. Many students could also correctly convert the structural formula into an empirical formula in Part (b) and then went on to correctly determine the percentage by mass of each element in Part (c), though sometimes only with the help of ECF. Writing the correct combustion equation was difficult for only the weaker candidates.

