| Date | May 2013 | Marks available | 2 | Reference code | 13M.2.sl.TZ2.4 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce and Show | Question number | 4 | Adapted from | N/A |
Question
Intermolecular forces are attractive forces between molecules.
Consider the compounds \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\) and \({\text{C}}{{\text{H}}_{\text{4}}}\).
Identify the intermolecular forces present in hydrogen iodide in the liquid state, HI(l).
Deduce the full structural formula for both compounds, showing all the bonds present.
\({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\)\(\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \)\({\text{C}}{{\text{H}}_{\text{4}}}\)
State and explain which compound can form hydrogen bonds with water.
Draw a diagram showing the resulting hydrogen bonds between water and the compound chosen in (ii).
Markscheme
van der Waals’/London/dispersion and dipole-dipole;
Allow abbreviations for van der Waals’ as vdW or for London/dispersion as FDL.
(CH3)2NH :
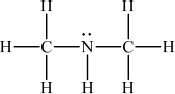 ;
;
Lone pair not necessary.
CH4 :
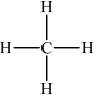 ;
;
All bonds (including CH bonds of methyl groups) must be shown for both structures.
Penalize missing hydrogens once only.
(CH3)2NH ;
(intermolecular) attraction between hydrogen (atom) in O–H/N–H (polar) bond and (lone pair on) electronegative N/O / hydrogen between two very electronegative elements (nitrogen and oxygen) / OWTTE;
Accept hydrogen bonded to nitrogen which is electronegative/has lone pair.
Do not allow ECF if M1 incorrect.
representative drawing showing hydrogen bond between (CH3)2NH and
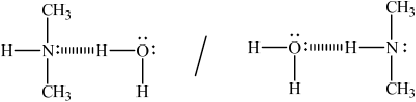
Do not penalize if lone pair as part of hydrogen bond is not shown.
Allow any representation of hydrogen bond (for example, dashed lines, dots etc.) which differs from full stick representation of the other covalent bonds in amine and water molecules.
Allow full line if labelled as hydrogen bond.
Lone pairs on oxygen not necessary.
Award mark if two hydrogen bonds drawn between the molecules from the lone pair and the H on the N.
Examiners report
Question 4 a) asked candidates to identify intermolecular forces in HI(l). A quick check of the Data Booklet should reveal an electronegativity difference of 0.5, so HI is polar and has dipole-dipole forces between molecules. Candidates should also be aware that the large number of electrons on iodine (large mass) would contribute to large van der Waals’ forces. Both answers were required for 1 mark. Many candidates only gave one response.
In b) (i) nearly all candidates could correctly draw the full structural formula of CH4 although some showed Lewis structures with dots and crosses. Fewer candidates could sketch the full structural formula of (CH3)2NH and drew the structure of ethylamine instead. Some candidates did not show all the bonds, leaving CH3 groups intact.
In b) (ii) candidates were asked which of these two compounds could form hydrogen bonds with water. A few did not realise that the question referred to the compounds already mentioned. This suggests that for some candidates their examination preparation has not included an understanding of question structures.
Most successfully identified (CH3)2NH but could not explain the hydrogen bond formation for the second mark. Many candidates then managed to draw a diagram of the hydrogen bonds, although some showed their lack of understanding of the nature of a hydrogen bond and drew them as covalent or dative covalent bonds.

