| Date | May 2018 | Marks available | 2 | Reference code | 18M.3.sl.TZ1.6 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Deduce | Question number | 6 | Adapted from | N/A |
Question
Insulin was the first protein to be sequenced. It was determined that the end of one chain had the primary structure Phe–Val–Asn–Gln.
Paper chromatography can be used to identify the amino acids in insulin.
Draw the structural formula of a dipeptide containing the residues of valine, Val, and asparagine, Asn, using section 33 of the data booklet.
Deduce the strongest intermolecular forces that would occur between the following amino acid residues in a protein chain.
State the name of the process used to break down the insulin protein into its constituent amino acids.
Outline how the amino acids may be identified from a paper chromatogram.
Markscheme
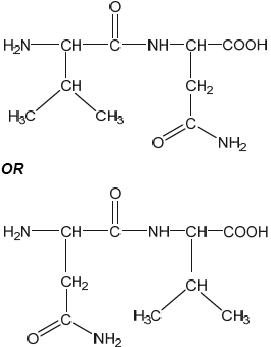
correct structures of Val AND Asn
correct amide link
[2 marks]
Phenylalanine and valine:
London/dispersion/instantaneous induced dipole-induced dipole forces
OR
permanent dipole-induced dipole «interactions»
Glutamine and asparagine:
hydrogen bonds
Do not accept dipole-dipole interactions.
[2 marks]
hydrolysis
[1 mark]
compare Rf with known amino acids
OR
compare distance moved with known amino acids
Accept “from Rf”.
[1 mark]

