| Date | May 2013 | Marks available | 1 | Reference code | 13M.3.sl.TZ2.B2 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Describe | Question number | B2 | Adapted from | N/A |
Question
Papain is a globular protein which is present in papaya fruit. Part of the sequence of its polypeptide chain is Gly–Cys–Val–Gly.
In the analysis of proteins, mixtures of amino acids with different isoelectric points can be separated using electrophoresis.
Proteins such as papain are formed by the condensation reactions of 2-amino acids.
By referring to Table 19 of the Data Booklet, draw the structural formulas of the two dipeptides formed by the reaction of glycine with cysteine.
Describe the essential features of electrophoresis.
Arginine, cysteine and glycine undergo electrophoresis at pH 6.0. Deduce which amino acid moves towards the positive electrode (anode).
Describe what is meant by the tertiary structure of proteins.
Identify two interactions which are responsible for this type of structure.
Markscheme
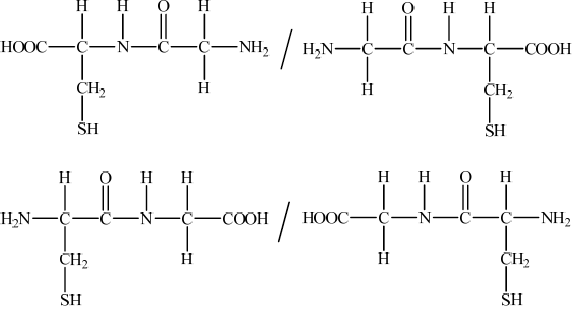
Accept condensed versions of structures such as –NH–CO–CH2– / –HN–CO–CH2–
Penalise repeated minor errors, such as incorrect representation of peptide bond (–COHN–/–NHOC–) once only.
Award [1] for a correct peptide link if the rest of the structure is incorrect.
sample of amino acids/mixture placed/spotted on gel/ polyacrylamide/PAGE/paper;
buffer solution / solution of known pH;
(high) potential (difference)/electric field/voltage applied / + and – electrodes/anode and cathode connected;
Accept current/electricity passed through.
different amino acids move different rates/distances according to their charge/isoelectric point / amino acids move towards oppositely charged electrode / OWTTE;
spray/develop with ninhydrin/organic dye/ detect by staining/fluorescence under UV light;
measure distance travelled and compare with standards/isoelectric points;
Award [1 max] for the statement “different amino-acids move to different extents”.
cysteine;
folding of secondary structure / produces 3D shape of the protein;
Do not accept “folding of the protein chain” / OWTTE.
hydrogen/H bonds
ionic bonds/attraction
van der Waals’/London/dispersion forces
disulfide bridges
hydrophobic interactions
Award [1] for any two of the above.
Examiners report
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.

