| Date | May 2010 | Marks available | 6 | Reference code | 10M.3.sl.TZ2.A3 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Calculate, Describe, Explain, and State | Question number | A3 | Adapted from | N/A |
Question
Paper chromatography may be used to separate a mixture of sugars.
(a) State the stationary phase and an example of a mobile phase used in paper chromatography.
Stationary phase:
Mobile phase:
(b) The identity of two sugars in a mixture can be determined by measuring their \({R_{\text{f}}}\) values, after staining.
(i) Describe how an \({R_{\text{f}}}\) value can be calculated.
(ii) Calculate the \({R_{\text{f}}}\) value for sugar 2 in the chromatogram below.
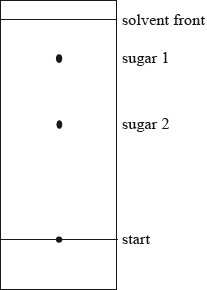
(c) Explain how the \({R_{\text{f}}}\) value of sugar 2 could be used to identify it.
Markscheme
(a) Stationary phase:
water in the paper;
Mobile phase:
water / any other non corrosive solvent or solvent mixture;
(b) (i) \({R_{\text{f}}} = \frac{{{\text{distance moved by substance}}}}{{{\text{distance moved by solvent / solvent front}}}}\);
(ii) \({R_{\text{f}}} = \frac{{3.0}}{{5.7}} = 0.53\);
Accept answers between 0.5 and 0.55 (± 1mm on measurements).
(c) compare \({R_{\text{f}}}\) of unknown to known values;
(conducted) under the same conditions (of stationary/mobile phase);
Allow second mark for specifying a particular condition to keep the same (e.g. solvent).
Examiners report
This was generally well done with most candidates displaying a good comprehension of the \({R_{\text{f}}}\) concept. In Part (c) many candidates correctly suggested comparing the \({R_{\text{f}}}\) value to that of standard samples, but very few pointed out that these must be obtained under identical conditions.

