| Date | May 2014 | Marks available | 2 | Reference code | 14M.3.sl.TZ2.2 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Calculate and Outline | Question number | 2 | Adapted from | N/A |
Question
Thin-layer chromatography (TLC) is an example of adsorption chromatography.
A mixture of two organic compounds was separated by TLC using a non-polar solvent.
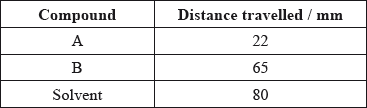
(i) Calculate the \({R_{\text{f}}}\) values of A and B.
(ii) Outline why compound B has travelled the greater distance.
Markscheme
(i) 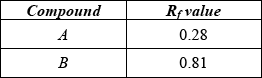 ;
;
Award [1] for both correct.
(ii) B is more soluble in solvent/mobile phase / B is less polar than A / B is less strongly adsorbed onto stationary phase;
Accept B is non-polar.
Do not allow “greater attraction/affinity to solvent” without reference to solubility.
Examiners report
(i) Very well answered. Many candidates used an inappropriate number of significant figures for the calculated \({R_{\text{f}}}\) values, but this was not penalized in this instance.
(ii) Generally well answered. Some candidates successfully related the solubility of compound B to its polarity.
Syllabus sections
- 17N.3.sl.TZ0.11: Enzyme activity depends on many factors. Explain how pH change causes loss of activity of an...
- 17M.3.hl.TZ2.8c.i: An aqueous buffer solution contains both the zwitterion and the anionic forms of alanine....
- 17M.3.sl.TZ2.7d: A mixture of the three amino acids, cysteine, glutamine and lysine, was placed in the centre...
- 17M.3.sl.TZ2.7c: Deduce the structural formula of the predominant form of cysteine at pH 1.0.
- 17M.3.sl.TZ2.7b: Identify the type of bond between two cysteine residues in the tertiary structure of a protein.
- 17M.3.sl.TZ2.7a: Deduce the structural formula of the dipeptide Cys-Lys.
- 17M.3.sl.TZ1.13c: The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and...
- 17M.3.sl.TZ1.13b.ii: Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
- 17M.3.sl.TZ1.13b.i: Identify the name of the amino acid that does not move under the influence of the applied...
- 16N.3.sl.TZ0.10d: The fibrous protein keratin has a secondary structure with a helical arrangement. (i) State...
- 16N.3.sl.TZ0.10c: Determine the number of different tripeptides that can be made from twenty different amino...
- 16N.3.sl.TZ0.10b: A mixture of amino acids is separated by gel electrophoresis at pH 6.0. The amino acids are...
- 16M.3.hl.TZ0.10c: (i) Serine is a chiral amino acid. Draw both enantiomers of serine. (ii) State the...
- 16M.3.sl.TZ0.10d: Bioplastics are broken down by enzyme catalysed reactions. Sketch a graph illustrating how...
- 16M.3.sl.TZ0.9b: A tripeptide, X, containing leucine (Leu), lysine (Lys) and glutamic acid (Glu) is hydrolysed...
- 16M.3.sl.TZ0.9a: Deduce the structures of the most abundant form of glycine in three buffer solutions at pH...
- 15M.3.hl.TZ1.8a: Outline the relationship between enzyme activity and concentration of the substrate.
- 15M.3.hl.TZ2.6b: (i) Pepsin is a protein which functions as an enzyme in human stomachs. Describe the...
- 15M.3.sl.TZ1.4a.i: State why they are called 2-amino acids.
- 15M.3.sl.TZ1.4c: Proteins carry out a number of important functions in the body. State the function of collagen.
- 15M.3.sl.TZ1.4a.ii: Identify the amino acid with the empirical formula C3H7ON2
- 15M.3.sl.TZ1.4a.iv: Deduce the structure of valine in a solution with a pH of 4.0.
- 15M.3.sl.TZ1.4b: Deduce the primary structures of the tripeptides formed by reacting together one molecule of...
- 15M.3.sl.TZ2.5a.i: Explain how individual amino acids can be obtained from proteins for chromatographic separation.
- 15M.3.sl.TZ2.5a.ii: A mixture of amino acids was spotted onto chromatography paper and eluted with a solvent...
- 15M.3.sl.TZ2.5b: One protein found in the human body is collagen. Identify its function.
- 14M.3.sl.TZ1.3a: Calculate the \({R_{\text{f}}}\) values for the two spots. Spot 1: Spot 2:
- 14M.3.sl.TZ1.3b: Suggest a reason why only two spots are present.
- 14M.3.sl.TZ1.3c: Suggest how the chromatography experiment with the same sample could be altered in order to...
- 14M.3.sl.TZ2.17b: State two named functional groups present in each of the following molecules found in two...
- 14N.3.hl.TZ0.8: Describe three characteristics of enzymes.
- 14N.3.sl.TZ0.3: (a) State how you could tell whether the ink was a single substance or a mixture of...
- 14N.3.sl.TZ0.6a: Draw the structure of a 2-amino acid.
- 14N.3.sl.TZ0.6c: Explain how a given protein can be broken down into its constituent amino acids and how these...
- 13N.3.hl.TZ0.7a: State the structural formula of cysteine as a zwitterion.
- 13N.3.hl.TZ0.7b: With reference to the isoelectric points of alanine and cysteine, describe with a reason what...
- 13N.3.hl.TZ0.7c: Cysteine is responsible for a specific type of intra-molecular bonding within a protein...
- 13N.3.sl.TZ0.6a: State the structural formula of cysteine as a zwitterion.
- 13N.3.sl.TZ0.6b.i: identify a pH value where both amino acids would be positively charged.
- 13N.3.sl.TZ0.6b.ii: describe with a reason what pH value would be suitable to use in an electrophoresis...
- 13N.3.sl.TZ0.6c: Cysteine is responsible for a specific type of intra-molecular bonding within a protein...
- 13N.3.sl.TZ0.6d: State three functions of proteins in the body and include a named example for each.
- 13M.3.hl.TZ1.B4a.i: Describe the characteristics of an enzyme (such as catalase).
- 13M.3.sl.TZ1.B1a.i: Using Table 19 of the Data Booklet, deduce the structural formulas of the two dipeptides...
- 13M.3.sl.TZ1.B1b: Explain how amino acids can be analysed using electrophoresis.
- 13M.3.sl.TZ1.B1c: List two functions of proteins in the body.
- 13M.3.sl.TZ1.B1a.ii: State the other substance formed during this reaction.
- 13M.3.sl.TZ2.B2c.i: Describe what is meant by the tertiary structure of proteins.
- 13M.3.sl.TZ2.B2c.ii: Identify two interactions which are responsible for this type of structure.
- 13M.3.sl.TZ2.A1c: If the components of the mixture are coloured then they can be seen with the naked eye....
- 13M.3.sl.TZ2.A1a: State the meaning of the term retention factor.
- 13M.3.sl.TZ2.B2b.ii: Arginine, cysteine and glycine undergo electrophoresis at pH 6.0. Deduce which amino acid...
- 13M.3.sl.TZ2.A1b: Explain why the value of the retention factor for the same component can be very different if...
- 13M.3.sl.TZ2.B2a: Proteins such as papain are formed by the condensation reactions of 2-amino acids. By...
- 13M.3.sl.TZ2.B2b.i: Describe the essential features of electrophoresis.
- 09N.3.hl.TZ0.B2a: State the major function of enzymes in the human body.
- 09N.3.hl.TZ0.B2b: Describe the mechanism of enzyme action in terms of structure.
- 09N.3.sl.TZ0.B1e: Explain how a sample of a protein can be analysed by electrophoresis.
- 09N.3.sl.TZ0.B1a: State the general formula of 2-amino acids.
- 09N.3.sl.TZ0.B1b: State two characteristic properties of 2-amino acids.
- 09N.3.sl.TZ0.B1c: Using Table 19 of the Data Booklet, deduce the structural formula of two dipeptides that...
- 09N.3.sl.TZ0.B1d.i: Explain the difference between the primary and secondary structure of proteins.
- 09N.3.sl.TZ0.B1d.ii: State the predominant interaction responsible for the secondary structure.
- 10M.3.sl.TZ1.B1c: Deduce the structures of the two different dipeptides that can be formed when one molecule of...
- 10M.3.sl.TZ1.B1a: (i) a solution with a pH of 2. (ii) a solution with a pH of 12.
- 10M.3.sl.TZ1.B1b: Deduce the structure of serine at the isoelectric point.
- 10M.3.sl.TZ2.A3: (a) State the stationary phase and an example of a mobile phase used in paper...
- 10M.3.sl.TZ2.B2: (a) List four major functions of proteins in the human body. (b) Deduce the...
- 09M.3.hl.TZ1.B4a: Describe the characteristics of an enzyme such as pepsin, and compare its catalytic behaviour...
- 09M.3.sl.TZ1.B1b.ii: State the name of the bond or interaction that is responsible for the secondary structure.
- 09M.3.sl.TZ1.B1a: Describe the characteristic properties of 2-amino acids.
- 09M.3.sl.TZ1.B1b.i: State the name of the bond or interaction that is responsible for linking the amino acids...
- 09M.3.sl.TZ1.B1b.iii: State two of the bonds or interactions responsible for the 3D shape of myoglobin.
- 09M.3.hl.TZ2.B4a: Explain the relationship between enzyme activity and concentration of the substrate.
- 09M.3.sl.TZ2.A1c.iii: Calculate the \({R_{\text{f}}}\) of the amino acid.
- 11M.3.sl.TZ2.B2a.iii: The amino acid at spot B is at its isoelectric point. Describe one characteristic of an amino...
- 11M.3.sl.TZ2.B2a.i: Describe how the amino acid spots may have been developed.
- 11M.3.sl.TZ2.B2a.ii: Predict which amino acid is present at spot C. Explain your answer.
- 12M.3.hl.TZ1.B1f: Compare the behaviour of enzymes and inorganic catalysts, including reference to the...
- 12M.3.sl.TZ1.F1b: Identify the types of nutrients A, B and C. A B C
- 12M.3.hl.TZ2.B3a: State and explain how the rate of an enzyme-catalysed reaction is related to the substrate...
- 11N.3.hl.TZ0.B5b: State and explain the effect of increasing the temperature from 20 °C to 60 °C on an...
- 11N.3.hl.TZ0.B5a: Explain how enzymes, such as hexokinase, are able to catalyse reactions.
- 11N.3.sl.TZ0.B2a: State the name of the linkage that is broken during the hydrolysis of a protein and draw its...
- 11N.3.sl.TZ0.B2b: Explain how electrophoresis is used to analyse a protein.

