| Date | November 2013 | Marks available | 2 | Reference code | 13N.3.hl.TZ0.7 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Describe | Question number | 7 | Adapted from | N/A |
Question
Proteins are polymers of 2-amino acids. The structures of the common amino acids are given in Table 19 of the Data Booklet. This question refers to the two amino acids alanine and cysteine.
State the structural formula of cysteine as a zwitterion.
With reference to the isoelectric points of alanine and cysteine, describe with a reason what pH value would be suitable to use in an electrophoresis experiment designed to separate these two amino acids in solution.
Cysteine is responsible for a specific type of intra-molecular bonding within a protein molecule. State the name of this type of interaction and outline how it is different from other interactions responsible for the tertiary structure.
Markscheme
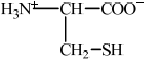 ;
;
Accept full or condensed structural formulas as long as correct charges on N/NH3 and O are represented.
Accept NH3+ for H3N+ in the diagram.
any value or range from 5.1– 6.0 ;
alanine positive and cysteine negative;
Accept biggest charge difference/opposite charges between isoelectric points so move in opposite directions.
Need reference to charges to score M2.
disulfide bridge;
Accept S–S.
covalent / strongest bond;
Examiners report
Option B was a very popular, and question 6 was well answered with the exception of not listing alkenyl when identifying two functional groups common to three vitamins (A, C and D). Some students did not read the question on formula of zwitterion carefully and instead have the formula of the amino acid itself without any charges. In the separation of alanine and cysteine, the first mark was well scored by many while the second mark proved to be more demanding and often candidates lost this mark as no reference was made to charges or charges inversely stated. Although the disulfide bridge was correctly identified by even weaker candidates a much few were able to identify this as a covalent bond. Structure of the triglyceride was better answered than in past sessions but drawing the ester linkage correctly was still challenging for many candidates. Although the identification of the other reactant (water) was identified by many, the one essential condition (enzyme/lipase) was done poorly. Identification of the polyunsaturated fatty acid was done well by most but the second mark on its ability to lower LDL cholesterol was missed by most. The question on enzymes and inorganic catalysts was done poorly since comparison was often missing. While some candidates were able to suggest a pair of ions in cytochrome oxidase, only stronger candidates provided both pairs. Competitive and non-competitive inhibition was generally well done; however, the reason why it is more likely that NO, rather than the cyanide ion, acts competitively was not done as well. The redox reaction of the reducing agent \({\text{X}}{{\text{H}}_{\text{2}}}\) with \({{\text{O}}_{\text{2}}}\) produced a range of possible equations but rarely did candidates scored full marks.
Option B was a very popular, and question 6 was well answered with the exception of not listing alkenyl when identifying two functional groups common to three vitamins (A, C and D). Some students did not read the question on formula of zwitterion carefully and instead have the formula of the amino acid itself without any charges. In the separation of alanine and cysteine, the first mark was well scored by many while the second mark proved to be more demanding and often candidates lost this mark as no reference was made to charges or charges inversely stated. Although the disulfide bridge was correctly identified by even weaker candidates a much few were able to identify this as a covalent bond. Structure of the triglyceride was better answered than in past sessions but drawing the ester linkage correctly was still challenging for many candidates. Although the identification of the other reactant (water) was identified by many, the one essential condition (enzyme/lipase) was done poorly. Identification of the polyunsaturated fatty acid was done well by most but the second mark on its ability to lower LDL cholesterol was missed by most. The question on enzymes and inorganic catalysts was done poorly since comparison was often missing. While some candidates were able to suggest a pair of ions in cytochrome oxidase, only stronger candidates provided both pairs. Competitive and non-competitive inhibition was generally well done; however, the reason why it is more likely that NO, rather than the cyanide ion, acts competitively was not done as well. The redox reaction of the reducing agent \({\text{X}}{{\text{H}}_{\text{2}}}\) with \({{\text{O}}_{\text{2}}}\) produced a range of possible equations but rarely did candidates scored full marks.
Option B was a very popular, and question 6 was well answered with the exception of not listing alkenyl when identifying two functional groups common to three vitamins (A, C and D). Some students did not read the question on formula of zwitterion carefully and instead have the formula of the amino acid itself without any charges. In the separation of alanine and cysteine, the first mark was well scored by many while the second mark proved to be more demanding and often candidates lost this mark as no reference was made to charges or charges inversely stated. Although the disulfide bridge was correctly identified by even weaker candidates a much few were able to identify this as a covalent bond. Structure of the triglyceride was better answered than in past sessions but drawing the ester linkage correctly was still challenging for many candidates. Although the identification of the other reactant (water) was identified by many, the one essential condition (enzyme/lipase) was done poorly. Identification of the polyunsaturated fatty acid was done well by most but the second mark on its ability to lower LDL cholesterol was missed by most. The question on enzymes and inorganic catalysts was done poorly since comparison was often missing. While some candidates were able to suggest a pair of ions in cytochrome oxidase, only stronger candidates provided both pairs. Competitive and non-competitive inhibition was generally well done; however, the reason why it is more likely that NO, rather than the cyanide ion, acts competitively was not done as well. The redox reaction of the reducing agent \({\text{X}}{{\text{H}}_{\text{2}}}\) with \({{\text{O}}_{\text{2}}}\) produced a range of possible equations but rarely did candidates scored full marks.

