| Date | November 2009 | Marks available | 2 | Reference code | 09N.3.sl.TZ0.B1 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | State | Question number | B1 | Adapted from | N/A |
Question
Proteins are vital components of living systems.
State the general formula of 2-amino acids.
State two characteristic properties of 2-amino acids.
Using Table 19 of the Data Booklet, deduce the structural formula of two dipeptides that could be formed by the reaction of alanine with serine and state the other product of the reaction.
Other product of the reaction:
Explain the difference between the primary and secondary structure of proteins.
State the predominant interaction responsible for the secondary structure.
Explain how a sample of a protein can be analysed by electrophoresis.
Markscheme
H2NCHRCOOH;
Allow various other combinations e.g. RCH(NH2)COOH etc. and allow NH2 and HOOC on right etc.
Allow structural formula if drawn, showing all the bonds.
Do not accept the formula of a specific amino acid.
isoelectric point;
formation of zwitterion/inner salt / \({{\text{H}}_{\text{3}}}{{\text{N}}^ + }{\text{CHRCO}}{{\text{O}}^ - }\);
(can act as a) buffer / has both acidic and basic properties / can react with \({{\text{H}}^ + }\) or \({\text{O}}{{\text{H}}^ - }\) / can exist as cations in acidic solution and anions in alkaline solution;
can form proteins/dipeptides/peptides / can react to form condensation products;
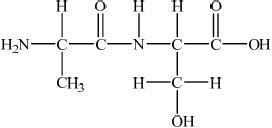 ;
;
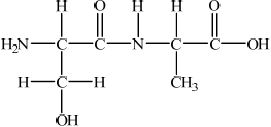 ;
;
Allow condensed structural formulas.
water/ H2O;
Primary structure:
(linear) sequence/order of amino acids / OWTTE;
Secondary structure:
way in which chain of amino acids folds itself / way in which sequence is kept together by hydrogen bonding between atoms in sequence / OWTTE;
Accept can exist as \(\alpha \)-helix or \(\beta \)-sheet.
hydrogen bonding/ H- bonding;
add hydrochloric acid/HCl / hydrolyse to convert protein into amino acid mixture / (successively) release amino acids;
mixture/amino acids spotted/placed on paper/gel;
Can be shown with diagram.
Do not accept protein placed/spotted on paper/gel.
use of buffer solution;
apply voltage/potential difference;
Can be shown with diagram.
Do not allow “pass current/electricity through mixture”.
amino acids move in different directions (depending on their isoelectric points);
develop with ninhydrin/triketohydrindane hydrate/2,2-dihydroxyindane-1,3-dione/organic dye;
measure distances moved / compare with known samples / measure isoelectric points (and compare with data);
Examiners report
Most attempts at (a) were successful.
A surprising number of candidates failed in (b) to give properties, as they misinterpreted the question and quoted structural features, such as the presence of an amino group.
Some students had difficulty deducing the structure of the two dipeptides in (c), however most candidates were able to identify the other product as water.
Most answers in (d) showed an understanding of protein primary and secondary structures.
Most answers in (d) showed an understanding of protein primary and secondary structures.
Responses to (e) were better than in some previous sessions; even so, full marks were rare, with many either omitting the hydrolysis of the protein or referring to a current being passed through the sample rather than a voltage being applied.

