| Date | May 2018 | Marks available | 2 | Reference code | 18M.3.hl.TZ2.8 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Draw | Question number | 8 | Adapted from | N/A |
Question
Amino acids are the building blocks of proteins.
Draw the structures of the main form of glycine in buffer solutions of pH 1.0 and 6.0.
The pKa of glycine is 2.34.
Calculate the pH of a buffer system with a concentration of 1.25 × 10−3 mol dm−3 carbonic acid and 2.50 × 10−2 mol dm−3 sodium hydrogen carbonate. Use section 1 of the data booklet.
pKa (carbonic acid) = 6.36
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
UV-Vis spectroscopy can be used to determine the unknown concentration of a substance in a solution.
Calculate the concentration of an unknown sample of pepsin with an absorbance of 0.725 using section 1 of the data booklet.
Cell length = 1.00 cm
Molar absorptivity (extinction coefficient) of the sample = 49650 dm3 cm−1 mol−1
A different series of pepsin samples is used to develop a calibration curve.
Estimate the concentration of an unknown sample of pepsin with an absorbance of 0.30 from the graph.
Markscheme
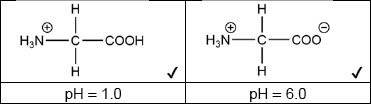
Penalize charge on incorrect atom once only.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
Accept condensed structural formulas.
Accept skeletal structures.
[2 marks]
ALTERNATIVE 1
«pH = 6.36 + log \(\left( {\frac{{2.50 \times {{10}^{ - 2}}}}{{1.25 \times {{10}^{ - 3}}}}} \right)\) =»
7.66
ALTERNATIVE 2
«Ka = 4.4 × 10–7 = [H+] \(\left( {\frac{{2.50 \times {{10}^{ - 2}}}}{{1.25 \times {{10}^{ - 3}}}}} \right)\), [H+] = 2.2 × 10–8 mol dm–3»
«pH =» 7.66
Do not accept “«pH =» 8”.
[1 mark]
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
Wedges AND dashes must be used.
[1 mark]
«\(\frac{{0.725}}{{49650{\text{ d}}{{\text{m}}^3}\;{\text{c}}{{\text{m}}^{ - 1}}\;{\text{mo}}{{\text{l}}^{ - 1}} \times 1.00\,{\text{cm}}}} = \)» 1.46 × 10−5 «mol dm−3»
[1 mark]
0.65 «μg cm–3»
Accept any value in the range 0.60–0.70 «μg cm–3»
[1 mark]

