| Date | November 2013 | Marks available | 2 | Reference code | 13N.3.sl.TZ0.6 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Outline and State | Question number | 6 | Adapted from | N/A |
Question
Proteins are polymers of 2-amino acids. The structures of the common amino acids are given in Table 19 of the Data Booklet. This question refers to the two amino acids alanine and cysteine.
With reference to the isoelectric points of alanine and cysteine:
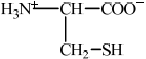
State the structural formula of cysteine as a zwitterion.
identify a pH value where both amino acids would be positively charged.
describe with a reason what pH value would be suitable to use in an electrophoresis experiment designed to separate these two amino acids in solution.
Cysteine is responsible for a specific type of intra-molecular bonding within a protein molecule. State the name of this type of interaction and outline how it is different from other interactions responsible for the tertiary structure.
State three functions of proteins in the body and include a named example for each.
Markscheme
 ;
;
Accept full or condensed structural formulas as long as correct charges on N/NH3 and O are represented.
Accept NH3+ for H3N+ in the diagram.
any value or range below 5.1;
any value or range from 5.1-6.0;
alanine positive and cysteine negative;
Accept biggest charge difference/opposite charges between isoelectric points so move in opposite directions.
Need reference to charges to score M2.
disulfide bridge;
Accept S–S.
covalent / strongest bond;
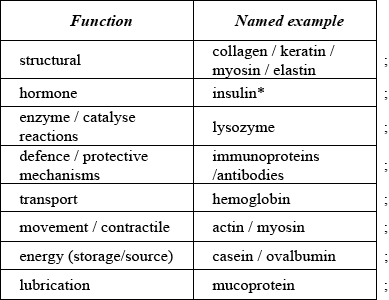
Need function with valid example for each mark.
Award [1 max] for three correct functions or three named examples.
Accept other correct examples.
Do not apply list principle.
* Other protein hormones include human growth hormone, follicle stimulating hormone (FSH), adrenocorticotropic hormone (corticotropin or ACTH), thyroid stimulating hormone (TSH).
* Do not accept steroids, sex hormones, testosterone, progesterone, estrogen, adrenalin.
Examiners report
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.

