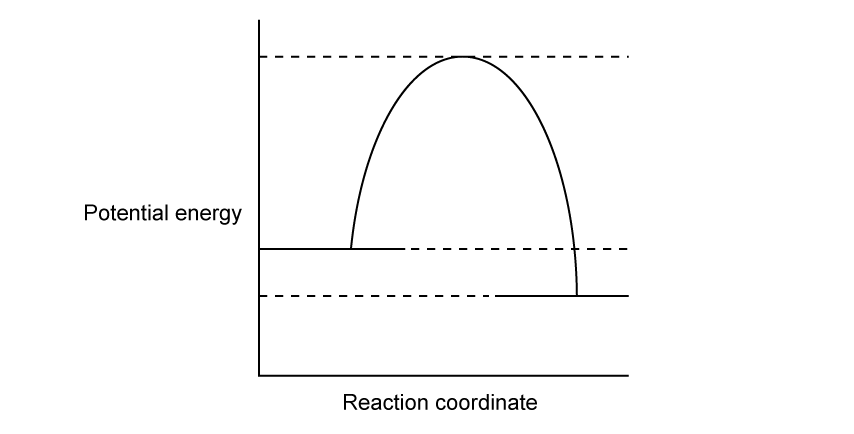
a)
State three ways of monitoring concentration changes in a reaction.
[3]
b)
A reaction is monitored by measuring the volume of a gas produced every 10 seconds. State an appropriate unit to use.
[1]
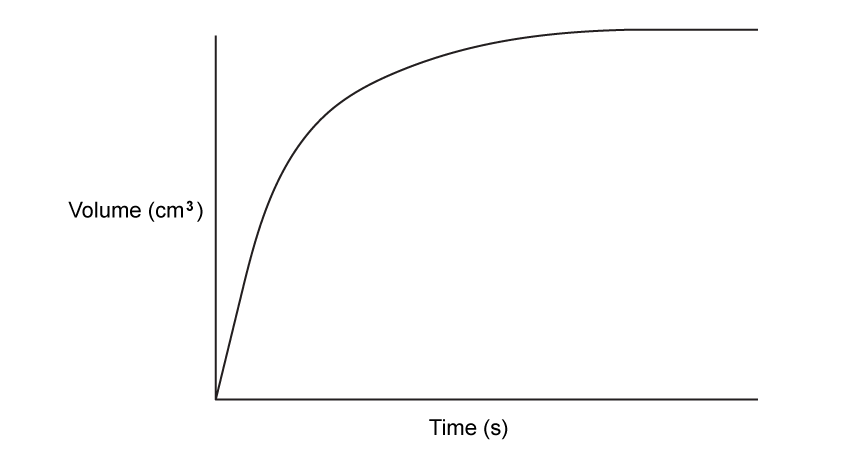
c)
Sketch a graph to show the volume of gas produced during the course of an experiment against the time taken.
[4]
d)
State the effect that increasing concentration has on the rate of a reaction.
[1]
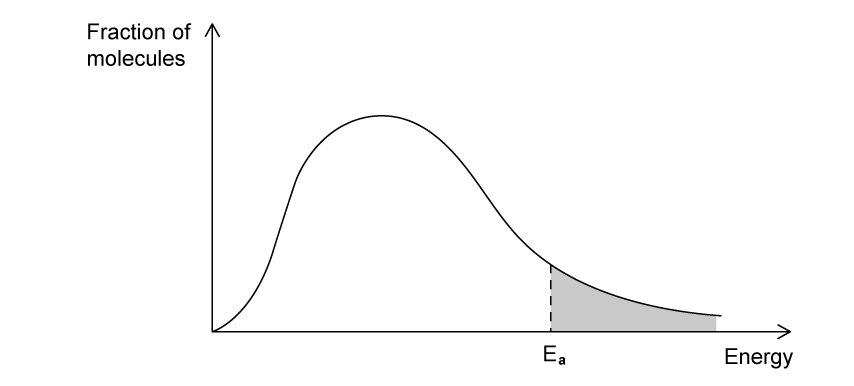
a)
State the effect that increasing temperature has on the rate of a reaction.
[1]
b)
Sketch a line on the graph to show the same reaction occurring at a higher temperature.
[3]
c)
State two variables that need to be controlled when investigating the effect of temperature on rate in the following reaction:
2HCl (aq) + Mg (s)
format('truetype')%3Bfont-weight%3Anormal%3Bfont-style%3Anormal%3B%7D%3C%2Fstyle%3E%3C%2Fdefs%3E%3Ctext%20font-family%3D%22math12bba9f4124283edd644799e0ce%22%20font-size%3D%2216%22%20text-anchor%3D%22middle%22%20x%3D%2210.5%22%20y%3D%2216%22%3E%26%23x2192%3B%3C%2Ftext%3E%3C%2Fsvg%3E)
MgCl
2 (aq) + H
2 (g)
[2]
d)
Suggest an appropriate piece of equipment to use to measure the volume of H2 gas produced in the reaction between HCl and Mg.
[1]