a)
Name the six changes of state, and state which changes are accompanied by a decrease in particle separation distances.
Assess your score
View Answer
b)
State the difference between a homogeneous and a heterogeneous mixture.
Assess your score
View Answer
c)
Classify the following mixtures as homogeneous or heterogeneous : crude oil, concrete and brass.
Assess your score
View Answer
d)
Which technique would be the most suitable for the separation of crude oil?
Assess your score
View Answer
Previous Question Next Question
a)
A compound with Mr =104.07 contains 34.62 % carbon, 3.88 % hydrogen and 61.50 % oxygen by mass.
Calculate its empirical formula.
Assess your score
View Answer
b)
Calculate the molecular formula of the compound in part a).
Assess your score
View Answer
c)
Draw a possible structure for the compound in part b).
Assess your score
View Answer
d)
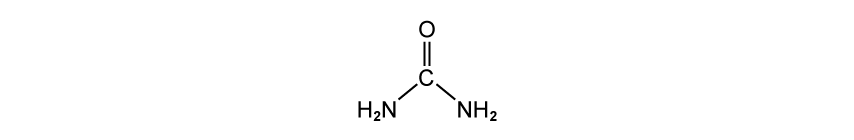
Deduce the empirical formula of the following molecule:
Assess your score
View Answer
Previous Question Next Question
c)
Determine the molecular formula of the compound in part b), given the Mr = 90.09.
Assess your score
View Answer
a)
State the meaning of the term empirical formula.
Assess your score
View Answer
An unknown compound contains carbon, hydrogen and oxygen only. It was shown to contain 3.20 g carbon, 0.54 g hydrogen and 4.26 g oxygen.
b)
Calculate the empirical formula of the unknown compound.
Assess your score
View Answer
Previous Question Next Question