a)
Deduce the missing information using section 5 of the data booklet, and complete the following table.
Symbol Protons Neutrons Electrons
23 Na
32 S2-
86 Sr2+
Assess your score
View Answer
b)
A sample of Rh contains the following isotopes. Calculate the relative atomic mass of Rh in the sample. Give your answer to 2 dp.
Isotope % Abundance
101 Rh85
102 Rh15
Assess your score
View Answer
c)
Deduce the number of protons, neutrons and electrons in an atom of 102 Rh.
Assess your score
View Answer
d)
Give the atomic symbol of an element which has more protons than neutrons. Use its most common isotope.
Assess your score
View Answer
Next Question
The atomic mass of each element in the periodic table is based on the carbon-12 scale.
a)
Describe the composition of the nucleus of carbon-12.
Assess your score
View Answer
b)
Carbon also exists as the isotope 14 C. How does the composition of this isotope differ from that of carbon-12.
Assess your score
View Answer
c)
The relative abundance of isotopes in a sample of carbon is 94% 12 C and 6% 14 C.
How would this information be obtained.
Assess your score
View Answer
d)
Calculate the relative atomic mass of the carbon sample in part c)
Assess your score
View Answer
Previous Question Next Question
a)
Describe what is meant by the term orbital.
Assess your score
View Answer
b)
Draw the shapes of the s, px, py and pz orbitals.
Assess your score
View Answer
c)
State the maximum number of orbitals in the n = 4 energy level.
Assess your score
View Answer
d)
List the d, f, p and s orbitals in order of decreasing energies.
Assess your score
View Answer
Previous Question Next Question
a)
Write the full electronic configurations for the following species
i)
K
ii)
Sr2+
Assess your score
View Answer
b)
Write the condensed electronic configurations for the following species
i)
Na
ii)
Al3 +
Assess your score
View Answer
c)
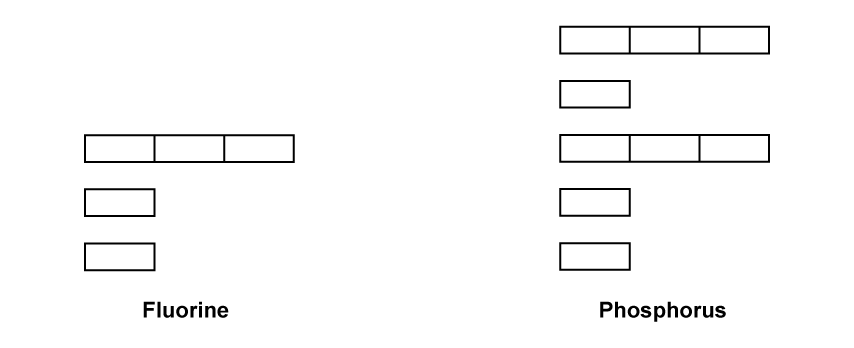
Complete the orbital diagrams of phosphorus and fluorine as shown in the diagram below.
Assess your score
View Answer
d)
Give the number of each type of orbital in the first four energy levels.
Assess your score
View Answer
Previous Question Next Question
a)
Using sections 1 and 3 of the data booklet describe how the following change in moving from the infrared region of the electromagnetic spectrum to the radio region of the electromagnetic spectrum.
i)
Wavelength
ii)
Frequency
iii)
Energy
Assess your score
View Answer
b)
Describe the process occurring in an atom to produce a single line on an emission spectrum.
Assess your score
View Answer
c)
Distinguish between a continuous spectrum and a line spectrum .
Assess your score
View Answer
d)
Describe the emission spectrum of hydrogen. Outline how this spectrum is related to the energy levels in the hydrogen atom.
Assess your score
View Answer
Previous Question