a)
During chemical reactions, enthalpy changes occur as bonds are broken and formed.
i)
Thermal energy is needed to overcome the attractive forces between atoms. In terms of thermal energy, name the process where bonds are broken.
ii)
When bonds are formed, thermal energy is released to the surroundings. In terms of thermal energy, name the process where bonds are made.
Assess your score
View Answer
b)
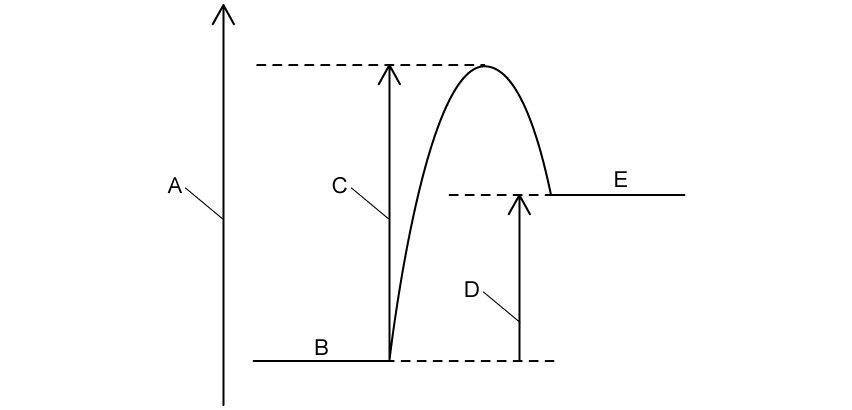
The energy level diagram for an endothermic reaction is shown below.
Complete the diagram by labelling parts A to E.
Assess your score
View Answer
c)
An element X undergoes complete combustion according to the following equation. The enthalpy change, ΔH , and activation energy, Ea , for this reaction are -520 kJ mol-1 and +630 kJ mol-1 respectively. Deduce whether this reaction is exothermic or endothermic.
X + O 2 → XO 2
Assess your score
View Answer
d)
Define the term average bond enthalpy.
Assess your score
View Answer
Next Question
a)
State the formula for calculating the standard enthalpy change of reaction, ΔH r
Assess your score
View Answer
b)
Use section 11 of the data booklet to calculate the enthalpy change, in kJ mol-1 , for the following reaction.
Cl2 + H2 → 2HCl
Assess your score
View Answer
c)
State whether the energy change for the reaction in part (b) is endothermic or exothermic.
Assess your score
View Answer
d)
Using section 11 of the data booklet, calculate the enthalpy change of reaction, ΔHr , in kJ mol-1 for the following reaction.
CH4 + Cl2 → CH3 Cl + HCl
Assess your score
View Answer
Previous Question Next Question
a)
Draw the Lewis structure of an oxygen molecule, O2 .
Assess your score
View Answer
b)
State the type of energy in the stratosphere responsible for the break down of the oxygen molecule.
Assess your score
View Answer
c)
State the equation for the formation of ozone and whether this reaction is endothermic or exothermic.
Assess your score
View Answer
d)
State the name of the type of compound that is responsible for the disruption of the temperature regulation in the stratosphere.
Assess your score
View Answer
Previous Question Next Question
a)
Using displayed formulae, write the equation for the reaction of ethene with water to form ethanol.
Assess your score
View Answer
b)
Using section 11 in the data booklet calculate the enthalpy change of reaction, ΔHr , for the reaction of ethene with water.
Assess your score
View Answer
c)
Define bond dissociation energy .
Assess your score
View Answer
Previous Question