Question 1a
a)
When anhydrous copper(II) sulfate is left in the atmosphere it will slowly turn to a blue pentahydrate solid. It is possible to measure the heat changes directly when both anhydrous and pentahydrated copper(II) sulfate are separately dissolved in water.
i)
Write an equation for the reaction of anhydrous copper(II) sulfate with water to form pentahydrated copper(II) sulfate.
[1]
ii)
Construct an energy cycle which can be used to determine the enthalpy change indirectly.
[2]
Question 1b
b)
To determine the enthalpy change a student placed 50 cm3 of water in a polystyrene cup and used a data logger to measure the temperature.
After two minutes she dissolved 6.30 g of anhydrous copper(II) sulfate in the water and continued to record the temperature while continuously stirring. She obtained the following results.
After two minutes she dissolved 6.30 g of anhydrous copper(II) sulfate in the water and continued to record the temperature while continuously stirring. She obtained the following results.
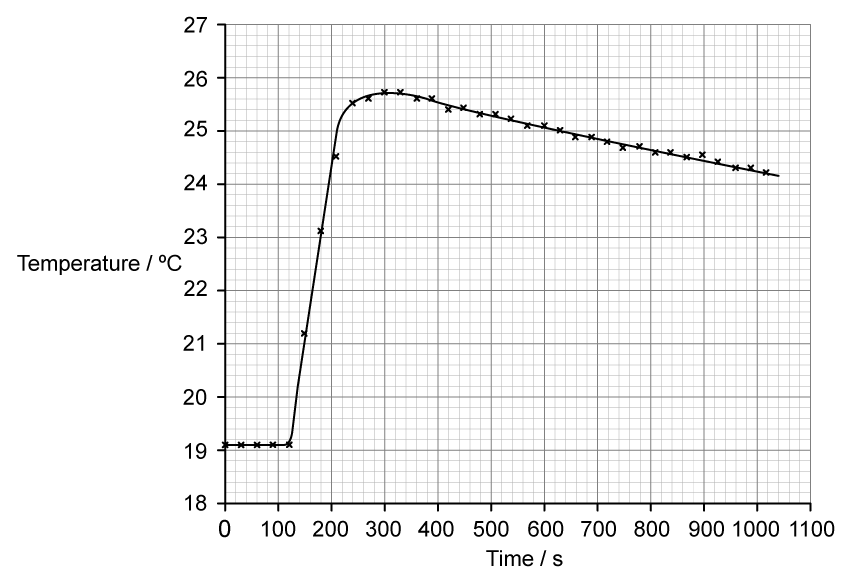
i)
Using section 6 in the data booklet, determine the amount, in moles, of copper(II) sulfate.
[1]
ii)
Determine the temperature change, in °C, for the reaction assuming no heat had been lost to the surroundings.
[1]
iii)
Using sections 1 and 2 in the data booklet, determine the heat change, in kJ mol-1, for the reaction.
[2]
Question 1c
c)
The student repeated the experiment using 7.83 g of pentahydrated copper(II) sulfate and observed the temperature decreased by 2.5 °C. The student used the same volume of water.
i)
Use section 6 of the data booklet to determine the amount, in moles, of pentahydrated copper(II) sulfate.
[1]
ii)
Use sections 1 and 2 in the data booklet to determine the heat change, in kJ mol-1.
[2]
Question 1d
d)
Use your answers to parts a), b) and c) to determine the energy change for dissolving copper(II) sulfate.
[2]