| Date | May 2009 | Marks available | 3 | Reference code | 09M.2.hl.TZ2.8 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Explain | Question number | 8 | Adapted from | N/A |
Question
In some countries, ethanol is mixed with gasoline (petrol) to produce a fuel for cars called gasohol.
Deduce a two-step synthesis for each of the following conversions. For each step, state the structural formulas of all reactants and products and state the conditions used in the reactions.
Ethanol to ethyl ethanoate.
Propene to propanone.
The reagents used in an elimination reaction are shown below.
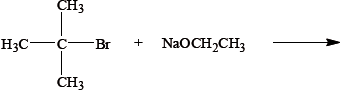
Explain the mechanism of this reaction using curly arrows to represent the movement of electron pairs.
Describe geometrical isomerism.
Draw the geometrical isomers of but-2-ene.
Draw the two enantiomers of butan-2-ol.
Markscheme
\[{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}\xrightarrow[{{{\text{H}}^ + }}]{{{{\text{K}}_2}{\text{C}}{{\text{r}}_2}{{\text{O}}_7}}}{\text{C}}{{\text{H}}_3}{\text{COOH}}\xrightarrow[{{{\text{H}}_2}{\text{S}}{{\text{O}}_4}}]{{{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}}}{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{O}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}}\]
Structural formulas of reactants and products
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH/C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{H}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{ (}} + {{\text{H}}_{\text{2}}}{\text{O)}}\);
Conditions/reagents used
reflux with named suitable acidified oxidising agent and then heat with alcohol and sulfuric acid;
Suitable oxidising agents are potassium dichromate/K2Cr2O7 / sodium dichromate/Na2Cr2O7 / dichromate/Cr2O72– / potassium manganate(VII)/potassium permanganate/KMnO4 / permanganate/manganate (VII)/MnO4–.
Accept H+/H2SO4 instead of sulfuric acid and acidified.
Award [1] for structural formulas of reactants and products and [1] for the correct conditions/reagents used.
\({{\text{H}}_{\text{2}}}{\text{C=CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}\xrightarrow[{{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(conc.)}}}]{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\xrightarrow[{{{\text{H}}^ + }}]{{{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}}}{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CO}}\)
Structural formulas of reactants and products
\({{\text{H}}_{\text{2}}}{\text{C=CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\) and \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CO}}\);
Conditions/reagents used
water/\({{\text{H}}_{\text{2}}}{\text{O}}\) and sulfuric acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\) / dilute acid medium and heat/reflux with suitable acidified oxidising agent;
Suitable oxidising agents are potassium dichromate/K2Cr2O7 / sodium dichromate/Na2Cr2O7 / dichromate/Cr2O72– / potassium manganate(VII)/potassium permanganate/KMnO4 / permanganate/manganate (VII)/MnO4–.
Accept H+/H2SO4 instead of acidified.
Note: If primary alcohol is given as product of first step, and everything else correct, award [1 max].
Accept either full or condensed structural formulas throughout (b).
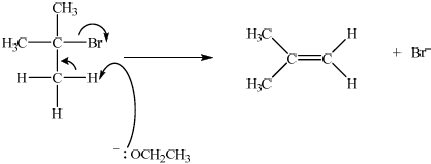
curly arrow going from O of \(^ - {\text{OC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) attacking hydrogen;
Allow the curly arrow to originate from either the lone pair or O of –OCH2CH3 but not from H of –OCH2CH3.
Do not award first mark if curly arrow originates from O of NaOCH2CH3.
curly arrow going from the C–H bond on the \(\beta \) carbon to the bond joining the \(\alpha \) carbon to the \(\beta \) carbon and curly arrow showing Br acting as leaving group;
formation of \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C=C}}{{\text{H}}_{\text{2}}}\) and \({\text{B}}{{\text{r}}^ - }\);
Allow formation of NaBr for third marking point, if NaOCH2CH3 was used (incorrectly) in the mechanism. Use of NaOCH2CH3 with curly arrow originating on O of NaOCH2CH3 is penalized already in the first marking point.
Accept alternative E1 type mechanism
curly arrow showing Br acting as leaving group to form carbocation;
curly arrow going from O of \(^ - {\text{OC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\) attacking hydrogen;
formation of \({({\text{C}}{{\text{H}}_{\text{3}}})_{\text{2}}}{\text{C=C}}{{\text{H}}_{\text{2}}}\) and Br–;
No marks awarded if a substitution mechanism is given.
compounds with the same (molecular formula and) structural formula but different arrangements of atoms in space / OWTTE;
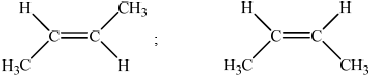 ;
;
Allow [1 max] if structures are correct but arrangement of groups in space does not clearly show the cis/ trans isomerism.
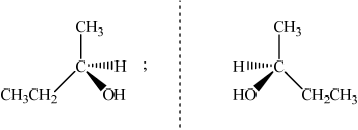 ;
;
Allow [1 max] if the structures are correct but it is not clear that they are mirror images.
Examiners report
In (b) (i) and (ii) only a few candidates answered both questions correctly. Fewer candidates scored one mark in one or both by correctly presenting the structural formulas. Conditions and reagents were in general poorly known.
In (b) (i) and (ii) only a few candidates answered both questions correctly. Fewer candidates scored one mark in one or both by correctly presenting the structural formulas. Conditions and reagents were in general poorly known.
Almost nobody answered (c) correctly. Candidates identified this as an SN1 mechanism. There were a number of G2 comments on this, and one respondent expressed surprise that sodium ethoxide was used as a reagent. However, the candidates were clearly told in the question that the reaction was an elimination reaction and hence should have been able to write the mechanism, as outlined in AS 20.3.2.
Part (d) (i), (ii) and (iii) were all well answered questions but some candidates lost a mark in (iii) because the structures were not represented as clear mirror images of each other.
Part (d) (i), (ii) and (iii) were all well answered questions but some candidates lost a mark in (iii) because the structures were not represented as clear mirror images of each other.
Part (d) (i), (ii) and (iii) were all well answered questions but some candidates lost a mark in (iii) because the structures were not represented as clear mirror images of each other.

