| Date | May 2010 | Marks available | 4 | Reference code | 10M.2.hl.TZ1.7 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Draw | Question number | 7 | Adapted from | N/A |
Question
Below are four structural isomers with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\). State the name of each of the isomers a, b, c and D.
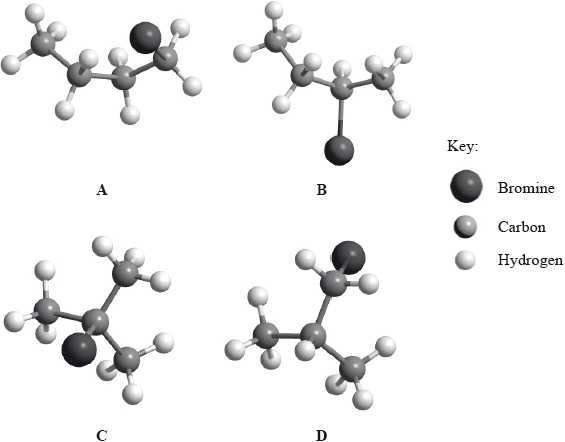
Identify the isomer(s) which will react with aqueous sodium hydroxide almost exclusively by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. State the meaning of the symbols in the term \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
Using the formula RBr to represent a bromoalkane, state an equation for the rate determining step of this \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction.
Identify one isomer that will react with aqueous sodium hydroxide almost exclusively by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Draw the mechanism for this reaction using curly arrows to represent the movement of electron pairs. Include the structural formulas of the transition state and the organic product.
State and explain how the rates of the reactions in parts (b) (i) and (b) (iii) are affected when the concentration of the sodium hydroxide is doubled.
State and explain how the rate of reaction of 1-bromobutane with sodium hydroxide compares with that of 1-chlorobutane with sodium hydroxide.
Identify the isomer of C4H9Br that can exist as stereoisomers. Outline how a polarimeter will distinguish between the isomers, and how their physical and chemical properties compare.
Markscheme
A: l-bromobutane;
B: 2-bromobutane;
C: 2-bromo-2-methylpropane;
D: 1-bromo-2-methylpropane;
Penalize incorrect punctuation, e.g. commas for hyphens, only once.
Accept 2-bromomethylpropane and 1-bromomethylpropane for C and D respectively.
C/2-bromo-2-methylpropane;
unimolecular nucleophilic substitution;
Accept first order in place of unimolecular.
\({\text{RBr}} \to {{\text{R}}^ + } + {\text{B}}{{\text{r}}^ - }\);
Allow use of 2-bromo-2-methylpropane instead of RBr.
A/1-bromobutane/D/1-bromo-2-methylpropane;
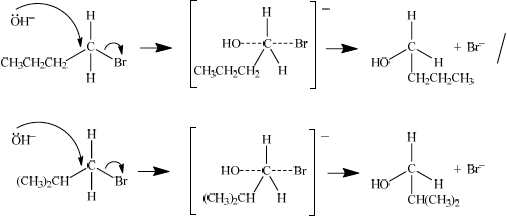
curly arrow going from lone pair/negative charge on O in \({\text{O}}{{\text{H}}^ - }\) to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 1-bromobutane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M4 if OH----C bond is represented.
(b) (i) no change as \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) does not appear in rate equation/in the rate determining step;
(b) (iii) rate doubles as the rate is proportional to \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) / \({\text{O}}{{\text{H}}^ - }\) appears in the ratedetermining/
slow step / first order with respect to \({\text{O}}{{\text{H}}^ - }\);
Award [1] if correctly predicts no rate change for SN1 and doubling of rate for SN2 of without suitable explanation.
rate of 1-bromobutane is faster;
C–Br bond is weaker/breaks more easily than C–Cl bond;
2-bromobutane/B;
(plane-) polarized light shone through;
enantiomers rotate plane of plane-polarized light to left or right/opposite directions (by same amount);
Accept “turn” instead of “rotate” but not “bend/reflect”.
physical properties identical (apart from effect on plane-polarized light);
chemical properties are identical (except with other chiral compounds);
Do not accept “similar” in place of “identical”.
Examiners report
This was the least popular question, but there were some very good answers seen. In parts (a) and (b), most candidates were able to correctly name the organic compounds, and identify which halogenoalkane would react via a SN1 or SN2 reaction.
Many candidates stated first order instead of unimolecular, which although we accepted it in this instance is not correct.
Attempts at the mechanism were generally disappointing though, with errors of incorrectly drawn arrows and faults in the transition state frequently occurring. Also candidates often had an arrow coming from an H in \({\text{O}}{{\text{H}}^ - }\) instead of from a lone pair of electrons on O.
Answers to (c) explaining how \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) effects rate were generally good, however, some only predicted and didn’t explain in terms of the rate limiting step.
Answers to (d) were generally good and only the weakest candidates didn‟t state that bromobutane reacted faster as the C-Br bond was weaker.
Most candidates in (e) knew how enantiomers affected plane-polarized light, but few stated that their properties were identical and many instead suggested they were similar.

