| Date | May 2014 | Marks available | 5 | Reference code | 14M.3.hl.TZ2.27 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Explain and Identify | Question number | 27 | Adapted from | N/A |
Question
(i) Identify the two reagents used to form the electrophile in the nitration of benzene.
(ii) Explain, using curly arrows to represent the movement of electrons, the mechanism for this reaction.
Markscheme
(i) \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\)/sulfuric acid and \({\text{HN}}{{\text{O}}_3}\)/nitric acid;
(ii) 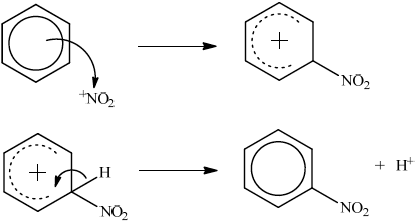
curly arrow going from delocalized electrons in benzene to \(^ + {\text{N}}{{\text{O}}_2}\);
Do not penalize if NO2+ is written.
representation of carbocation with correct formula and positive charge on ring;
curly arrow going from CH bond to benzene ring cation;
formation of organic product nitrobenzene and \({{\text{H}}^ + }\);
Allow mechanism with corresponding Kekulé structures.
Examiners report
Q27 was well answered by most candidates although some need to take care with the placement of the delocalized positive charge in the intermediate.

