| Date | May 2013 | Marks available | 1 | Reference code | 13M.2.hl.TZ1.9 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Identify | Question number | 9 | Adapted from | N/A |
Question
But-2-ene belongs to the homologous series of the alkenes.
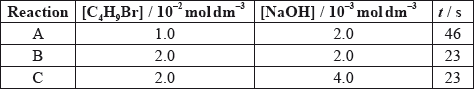
The time taken to produce a certain amount of product using different initial concentrations of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH is measured. The results are shown in the following table.
Outline three features of a homologous series.
Describe a test to distinguish but-2-ene from butane, including what is observed in each case.
2-bromobutane can be produced from but-2-ene. State the equation of this reaction using structural formulas.
State what is meant by the term stereoisomers.
Explain the existence of geometrical isomerism in but-2-ene.
Deduce the order of reaction with respect to \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH, using the data above.
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\)
NaOH:
Deduce the rate expression.
Based on the rate expression obtained in (c) (ii) state the units of the rate constant, \(k\).
Halogenalkanes can react with NaOH via \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) type mechanisms. Explain why \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) reacts via the mechanism described in (d) (i).
Identify the rate-determining step of this mechanism.
Markscheme
same functional group / same general formula;
difference between successive members is \({\text{C}}{{\text{H}}_{\text{2}}}\);
similar chemical properties;
Do not accept “same” chemical properties.
gradually changing physical properties;
adding bromine (water);
but-2-ene: brown/orange to colourless / decolourizes bromine water and
butane: does not change colour;
OR
adding acidified potassium permanganate solution/KMnO4(aq);
but-2-ene: purple to colourless/brown and
butane: does not change colour;
OR
adding Baeyer’s reagent;
but-2-ene: purple/pink to brown and
butane: does not change colour;
Do not accept “clear” or “transparent” for “colourless”.
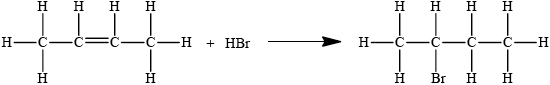
Accept condensed structural formula.
Penalise missing H atoms or incorrect bonds (such as C–HO, C–H2C) once only in the whole paper.
compounds with the same structural formula but different arrangement of atoms (in space);
(but-2-ene exists as) cis-but-2-ene and trans-but-2-ene /
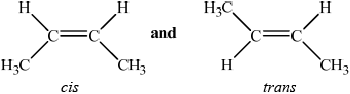 ;
;
restricted rotation of C=C/double bond;
C4H9Br:
[C4H9Br] doubles and time halves/rate doubles/rate proportional to [C4H9Br];
Do not accept rate increases when [C4H9Br] increases.
NaOH:
[NaOH] doubles and time/rate does not change/rate independent of [NaOH];
C4H9Br: first order and NaOH: zero order;
rate \( = k[{{\text{C}}_4}{{\text{H}}_9}{\text{Br}}]\);
Accept ECF.
\({{\text{s}}^{ - 1}}\);
Accept ECF.
greater stability of tertiary carbocation;
steric hindrance for \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism;
positive inductive effect (of alkyl groups);
Do not allow ECF.
the first step / \({\text{B}}{{\text{r}}^ - }\) leaving / formation of carbocation;
Do not allow ECF.
Examiners report
Features of an homologous series need to be learnt; this was answered relatively poorly.
The most common reagent was bromine (some indeed used liquid bromine!) and the common errors were using HBr and describing “colourless” as “clear”.
In (iii), some gave the equation backwards, a consequence, perhaps, of misreading the question.
In (iv) many referred to “same molecular formula” rather than “same structural formula”.
The lack of rotation about the double bond in (v) was not well described.
In (c) (i) the explanations were a little vague, some candidates perhaps being fooled by the data of time rather than rate. Many expected to be given marks for a series of numbers and calculations without explanations.
Answers to (ii) were usually consistent with (i).
Answers to (iii) were usually consistent with (i).
(ii) was rarely answered correctly while the answer to (iii) was patchy.
(ii) was rarely answered correctly while the answer to (iii) was patchy.

