| Date | November 2012 | Marks available | 4 | Reference code | 12N.2.sl.TZ0.6 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Explain | Question number | 6 | Adapted from | N/A |
Question
Alkenes, alcohols and esters are three families of organic compounds with many commercial uses.
Esters are often used in perfumes. Analysis of a compound containing the ester functional group only, gives a percentage composition by mass of C: 62.0% and H: 10.4%.
State the meaning of the term structural isomers.
X is an isomer of C4H8 and has the structural formula shown below.
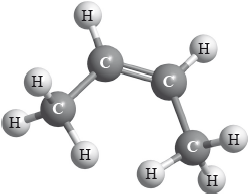
Apply IUPAC rules to name this isomer. Deduce the structural formulas of two other isomers of C4H8.
State the balanced chemical equation for the reaction of X with HBr to form Y.
Y reacts with aqueous sodium hydroxide, NaOH(aq), to form an alcohol, Z. Identify whether Z is a primary, secondary or tertiary alcohol.
Explain one suitable mechanism for the reaction in (v) using curly arrows to represent the movement of electron pairs.
Deduce the structural formula of the organic product formed when Z is oxidized by heating under reflux with acidified potassium dichromate(VI) and state the name of the functional group of this organic product.
Draw the ester functional group.
Determine the empirical formula of the ester, showing your working.
The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine its molecular formula.
Markscheme
compounds with the same molecular formula but different arrangement of atoms/structural formula/structures;
Do not allow similar instead of same.
(cis-)but-2-ene / (Z)but-2-ene / but-2-ene;
Accept (cis-)2-butene / Z-2-butene.
Ignore missing hyphens.
CH3CH2CH=CH2;
H2C=C(CH3)2;
Accept either full or condensed structural formulas.
Allow structural formula of trans-but-2-ene.
(CH3)CH=CH(CH3) + HBr \( \to \) CH3CHBrCH2CH3;
Allow C4H8 + HBr \( \to \) C4H9Br.
secondary/ 2°;
Since secondary could be either SN1 or SN2 so allow SN1 or SN2 for M1–M4.
SN1:
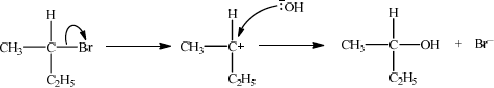
curly arrow showing Br leaving;
Do not allow arrow originating from C to C–Br bond.
representation of secondary carbocation;
curly arrow going from lone pair/negative charge on O in HO– to C+;
Do not allow arrow originating on H in HO–.
formation of organic product CH3CH(OH)C2H5/C4H9OH and Br–;
Allow formation of NaBr instead of Br–.
OR
SN2:
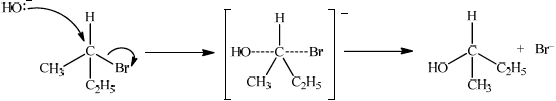
curly arrow going from lone pair/negative charge on O in HO– to C;
Do not allow curly arrow originating on H in HO–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 2-bromobutane or in the transition state.
Do not allow arrow originating from C to C–Br bond.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH ---- C bond is represented.
formation of organic product CH3CH(OH)C2H5/C4H9OH and Br–;
Allow formation of NaBr instead of Br–.
For primary Z from (v), for ECF SN2 required.
For tertiary Z from (v), for ECF SN1 required.
But curly arrow showing Br leaving and formation of C4H9OH and Br– can be scored for either mechanism (even if incorrect type).
For primary Z from (v) with 1-bromobutane stated in (vi), correct SN2 can score full marks.
If (v) is not answered and incorrect starting reagent is given in (vi), M1, M2 and M3 may be scored but not M4 for either correct SN1 or SN2.
CH3COCH2CH3;
Full or condensed structural formula may be given.
For primary Z from (v), accept CH3CH2CH2COOH/C3H7COOH but not CH3CH2CH2CHO.
ketone / alkanone;
drawing of \({\text{RCOOR'}}\) group / 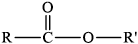
Allow C instead of R or \(R'\).
Allow 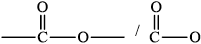
\((100 - 62.0 - 10.4 = ){\text{ }}27.6\% {\text{ O}}\);
\({n_C}:\left( {\frac{{62.0}}{{12.01}} = } \right){\text{ }}5.162{\text{ (mol)}}\) and \({n_H}:\left( {\frac{{10.4}}{{1.01}} = } \right){\text{ }}10.297{\text{ (mol)}}\)
and \({n_O}:\left( {\frac{{27.6}}{{16.00}} = } \right){\text{ }}1.725{\text{ (mol)}}\);
dividing 5.162 and 10.297 by 1.725 (to get values \({{\text{C}}_{{\text{2.992}}}}{{\text{H}}_{{\text{5.969}}}}{{\text{O}}_{\text{1}}}\));
(empirical formula =) \({{\text{C}}_3}{{\text{H}}_6}{\text{O}}\);
Award [4] for correct final answer if alternative method used.
Allow integer values for atomic masses (i.e. 12, 1 and 16).
C6H12O2;
Examiners report
Meaning of the term structural isomers was well defined with the weaker candidates referring to similar instead of same molecular formula but different arrangement of atoms.
Many candidates stated the IUPAC name of the isomers of C4H8 and deduced correctly the structural formulas of the two other isomers.
Most candidates were able to write the chemical equation for the reaction of the isomer of C4H8 with HBr and identify the alcohol formed by the reaction of that product with NaOH.
In part (a) (v), the mechanisms proved a problem for majority of candidates.
The use of curly arrows in reaction mechanisms continues to be poorly understood, the arrow often pointing in the wrong direction. Candidates must take care to accurately draw the position of the curly arrows illustrating the movement of electrons.
In part (b), the ester functional group was drawn correctly and it was pleasing to see that the majority of candidates handled the calculation of the empirical and molecular formulas extremely well.
In part (b), the ester functional group was drawn correctly and it was pleasing to see that the majority of candidates handled the calculation of the empirical and molecular formulas extremely well.
In part (b), the ester functional group was drawn correctly and it was pleasing to see that the majority of candidates handled the calculation of the empirical and molecular formulas extremely well.

