| Date | May 2011 | Marks available | 1 | Reference code | 11M.1.sl.TZ1.27 |
| Level | SL | Paper | 1 | Time zone | TZ1 |
| Command term | Determine | Question number | 27 | Adapted from | N/A |
Question
What is the type of mechanism and an important feature of the reaction between \({\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{Br}}\) and aqueous NaOH?
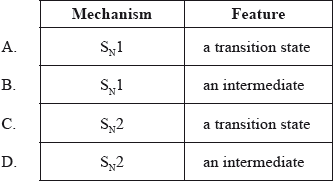
Markscheme
B
Examiners report
There were several comments on this question. One respondent stated that the terms were not covered in their IB textbook. It should be emphasised that it is the guide which defines the syllabus ONLY and NOT any one particular textbook which may be written for the programme itself. Other respondents stated that terms such as transition state and intermediate were not covered in the programme. However, this question relates to AS 10.5.2 and as such it is expected that some universally used terms would be introduced to candidates in explaining both \({{\text{S}}_{\text{N}}}\) mechanisms. The question certainly was very challenging for candidates and only 32.68% of candidates got the correct answer B.

