| Date | May 2011 | Marks available | 3 | Reference code | 11M.2.hl.TZ2.8 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Describe | Question number | 8 | Adapted from | N/A |
Question
There are several structural isomers with the molecular formula \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{Br}}\).
All the isomers react when warmed with a dilute aqueous solution of sodium hydroxide according to the equation below.
\[{{\text{C}}_5}{{\text{H}}_{11}}{\text{Br}} + {\text{NaOH}} \to {{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}} + {\text{NaBr}}\]
Deduce the name of one of the isomers which can exist as enantiomers and draw three-dimensional representations of its two enantiomers.
The reaction with 1-bromopentane proceeds by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
The reaction with 2-bromo-2-methylbutane proceeds by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
Explain why 1-bromopentane reacts by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism whereas 2-bromo-2-methylbutane reacts by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
Explain whether the boiling point of 1-bromopentane will be higher, lower or the same as that of 2-bromo-2-methylbutane.
The product \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) formed from the reaction with 1-bromopentane is warmed with ethanoic acid in the presence of a few drops of concentrated sulfuric acid. State the name of the type of reaction taking place and the structural formula of the organic product.
Markscheme
2-bromopentane 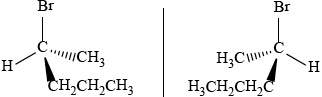
OR
1-bromo-2-methylbutane 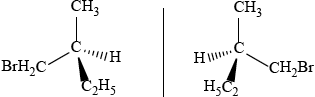
OR
2-bromo-3-methylbutane 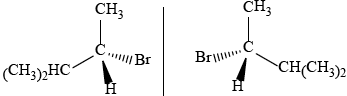
correct isomer 3D structure;
correct name;
correct enantiomer 3D structure;
If compound incorrectly named award [2 max] for two correct 3D enantiomers,
and [1 max] for a correct structure of an enantiomer not shown in 3D.
If non-optically active isomers given (e.g. 2-bromo-2-methyl-butane) award [1 max]
if name and 3D structure are correct.
Accept condensed form for alkyl chain throughout.
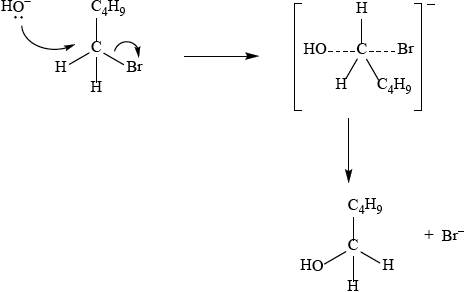
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C bonded to Br;
Do not allow curly arrow originating on H in \(H{O^ - }\) (e.g. originating on negative charge on H i.e. lone pair/negative charge must be on O).
curly arrow from C–Br bond to form \({\text{B}}{{\text{r}}^ - }\) (this can also be shown in transition state);
transition state showing overall negative charge;
Accept condensed formulas as long as curly arrows can still be shown e.g.
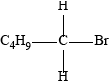
Accept 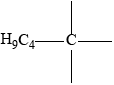
If wrong formula used for halogenoalkane, e.g. 1-bromobutane award [2 max].
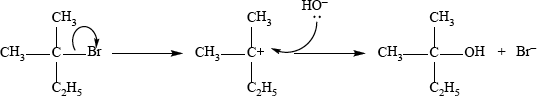
curly arrow from C–Br bond to form \({\text{B}}{{\text{r}}^ - }\);
correct structure of tertiary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
If non-bonding pair not shown then arrow must originate from negative sign on O or the minus sign.
Only penalize arrow from H once in (b).
If wrong formula is used for 2-bromo-2-methylbutane award [2 max].
the C bonded to the Br in 1-bromopentane is also bonded to two H atoms so can accommodate five groups around it in the transition state / OWTTE;
the C bonded to the Br in 2-bromo-2-methylbutane has three other (bulky) groups bonded to it so cannot accommodate five groups around it in the transition state / OWTTE;
2-bromo-2-methylbutane forms a tertiary carbocation which is stabilized by the positive inductive effect of the three alkyl groups / OWTTE;
1-bromopentane would form a primary carbocation (if it went by \({{\text{S}}_{\text{N}}}{\text{2}}\)) which is much less stable as there is only one alkyl group exerting a positive inductive effect / OWTTE;
the boiling point of 1-bromopentane is higher than the boiling point of 2-bromo-2-methylbutane;
2-bromo-2-methylbutane is more spherical in shape / less surface area in contact between molecules of 2-bromo-2-methylbutane than between molecules of 1-bromopentane / OWTTE;
hence weaker intermolecular forces of attraction/van der Waals’ forces of attraction between molecules of 2-bromo-2-methylbutane / OWTTE;
esterification / condensation;
\({\text{C}}{{\text{H}}_3}{\text{–CO–O–(C}}{{\text{H}}_2}{{\text{)}}_4}{\text{C}}{{\text{H}}_3}/{\text{C}}{{\text{H}}_3}{\text{COO(C}}{{\text{H}}_2}{{\text{)}}_4}{\text{C}}{{\text{H}}_3}/\)
\({\text{C}}{{\text{H}}_3}{\text{COOC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}/\)
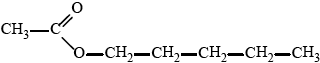 ;
;
Accept CH3–CO–O–C5H11
Examiners report
Although the least popular question, candidates were generally well prepared particularly in drawing enantiomers and describing the mechanisms for the two nucleophilic substitution reactions.
The representation of the \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms using curly arrows has significantly improved from previous sessions but mistakes are still being made.
Common errors in the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism include the curly arrow originating from the H in the hydroxide ion instead of the lone pair on the oxygen and the omission of the negative charge or square brackets from the transition state.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.
It was also disappointing to see H–C bonds in the transition state and HO–C–Br angles of less than 180°. If a candidate fully understood that the attack must be on the opposite side from the leaving group than this type of mistake would not appear. Explanations of why primary halogenoalkanes undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) reactions and why primary structures favour \({{\text{S}}_{\text{N}}}{\text{1}}\) reactions in terms of steric hindrance and carbocation stability were often incomplete with few candidates gaining full marks. Students should note that when asked to compare two molecules, their answers should refer explicitly to both; i.e. they had to mention that a tertiary compound halogenoalkane did have steric hindrance and a primary compound did not have steric hindrance. Some candidates also struggled to gave a full explanation of the higher boiling point of 1-bromopentane in terms of the greater surface contact between neighbouring molecules. Most candidates were familiar with the esterification reaction and able to give the structural formula of pentyl ethanoate. The prediction of the organic products of the elimination reaction proved to be beyond many, as candidates struggled to apply their knowledge in an unfamiliar context. Similarly, many were unable to give the equation for the condensation polymerisation reaction between benzene-1,4-dicarboxylc acid and pentane-1,5-diol. A significant number of students misread the question and attempted to describe a reaction between the acid and 1,5-dibromopentane instead.

