| Date | May 2010 | Marks available | 4 | Reference code | 10M.3.sl.TZ1.G2 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | G2 | Adapted from | N/A |
Question
Alkenes can undergo electrophilic addition reactions with bromine and with hydrogen bromide.
Name the product formed when but-2-ene reacts with
Explain how a bromine molecule is able to act as an electrophile.
(i) bromine.
(ii) hydrogen bromide.
When but-1-ene reacts with hydrogen bromide, two possible organic products could be formed but in practice only one organic product is obtained in high yield. Explain the mechanism for this reaction using curly arrows to represent the movement of electron pairs and explain clearly why only one organic product is formed.
Markscheme
as the bromine approaches the alkene an induced dipole is formed / OWTTE;
(i) 2,3-dibromobutane;
(ii) 2-bromobutane;
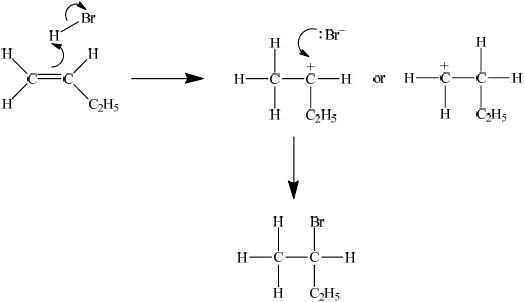
showing curly arrow from double bond to H (in H–Br) and curly arrow from bond in H–Br to Br;
showing the curly arrow from the lone pair/negative charge on Br– to the secondary carbocation and 2-bromobutane as correct product;
stating that the secondary carbocation will be formed in preference to the primary carbocation;
the two positive/electron releasing inductive effects due to the two R– groups on the secondary carbocation make it more stable;
Examiners report
In part (a) candidates rarely explained the induced dipole in the bromine molecule which allows it to act as an electrophile.
Part (b) was answered more effectively with many candidates correctly naming products formed from but-2-ene, although several candidates omitted ‘di’ from 2,3-dibromobutane and thus lost the mark.
The poor use of curly arrows was again evident in part (c) although some candidates clearly explained why only one organic product is formed when but-1-ene reacts with hydrogen bromide.

