| Date | May 2010 | Marks available | 4 | Reference code | 10M.2.sl.TZ1.6 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Explain and State | Question number | 6 | Adapted from | N/A |
Question
In an experiment to measure the enthalpy change of combustion of ethanol, a student heated a copper calorimeter containing 100 cm3 of water with a spirit lamp and collected the following data.
\[\begin{array}{*{20}{l}} {{\text{Initial temperature of water:}}}&{{\text{20.0 }}^\circ {\text{C}}} \\ {{\text{Final temperature of water:}}}&{{\text{55.0 }}^\circ {\text{C}}} \\ {{\text{Mass of ethanol burned:}}}&{{\text{1.78 g}}} \\ {{\text{Density of water:}}}&{{\text{1.00 g}}\,{\text{c}}{{\text{m}}^{ - 3}}} \end{array}\]
(i) Use the data to calculate the heat evolved when the ethanol was combusted.
(ii) Calculate the enthalpy change of combustion per mole of ethanol.
(iii) Suggest two reasons why the result is not the same as the value in the Data Booklet.
Ethanol is part of the homologous series of alcohols. Describe two features of a homologous series.
(i) Below are four structural isomers of alcohols with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). State the name of each of the isomers a, b, c and D.
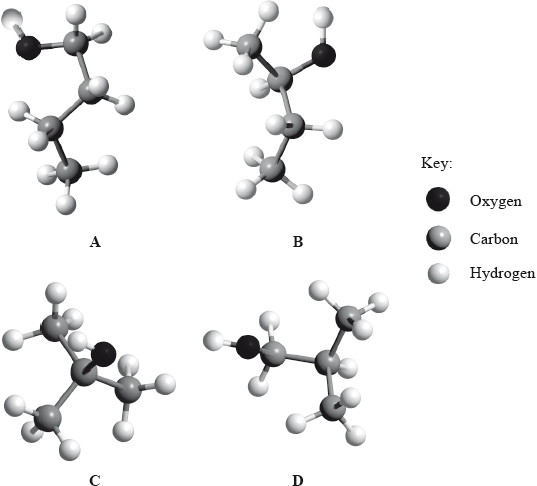
(ii) Determine the isomer that cannot be oxidized by acidifi ed potassium dichromate(VI), \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\).
(iii) Determine the isomer which can be oxidized to butanal.
(iv) Determine the isomer which can be oxidized to butanone.
(v) Suggest the structural formula of another isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\).

(i) Isomer a is formed by reacting 1-bromobutane with aqueous sodium hydroxide. State whether the reaction would proceed via an SN1 or SN2 mechanism.
(ii) Explain the mechanism named in part (d) (i) using curly arrows to represent the movement of electron pairs.
Markscheme
(i) \(100 \times 4.18 \times 35.0\);
14630 J / 14600 J / 14.6 kJ;
Award [2] for correct final answer.
No ECF here if incorrect mass used.
(ii) \(\frac{{1.78}}{{46.08}} = 0.0386{\text{ mol}}\);
\(\frac{{14.6}}{{0.0386}} = ( - )378{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
Accept (–)377 and (–)379 kJ\(\,\)mol–1.
Award [2] for correct final answer.
(iii) heat loss;
incomplete combustion;
heat absorbed by calorimeter not included;
Accept other sensible suggestions.
same general formula;
same functional group;
successive members differ by CH2;
Allow methylene for CH2.
similar chemical properties;
gradually changing physical properties;
(i) A: butan-1-ol;
B: butan-2-ol;
C: (2-)methylpropan-2-ol;
D: (2-)methylpropan-1-ol;
Accept answers in the form of 1-butanol and 2-methyl-2-propanol etc.
Penalize incorrect punctuation, e.g. commas for hyphens, only once.
(ii) C/(2-)methylpropan-2-ol;
(iii) A/butan-1-ol;
(iv) B/butan-2-ol;
(v) 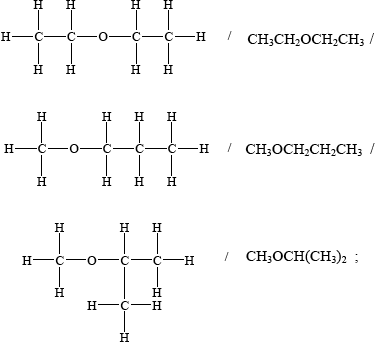
(i) SN2;
(ii) 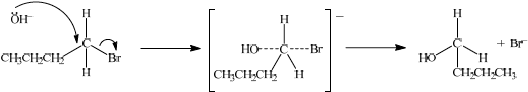
curly arrow going from lone pair/negative charge on O in \({\text{O}}{{\text{H}}^ - }\) to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in 1-bromobutane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH----C bond is represented.
Examiners report
This was the least popular of the Section B questions. (a) (i) was poorly answered. Many candidates had no idea and some candidates used the mass of ethanol instead of water. A few calculated correctly but failed to convert the mass of water to kg, or kJ to J, thereby ending up with the wrong unit for the answer. Only a small minority of candidates got (ii) correct. (iii) was well answered. Nearly all candidates referred to heat loss but only the better candidates were able to give a second reason.
Most candidates were able to describe two features of a homologous series in (b).
(c) was usually well done, but some candidates struggled with the structural formula of the ether isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\) in (v).
One G2 comment stated that the ether functional group is not listed as one of the formal functional groups in Topic 10, which is correct. However, this aspect has been asked previously on SL papers in relation to deducing specific isomers (rather than naming the ether group) and although candidates are not required to know that C-O-C is the ether functional group, there is an expectation that they should be able to deduce an isomer based on C-O-C, as this is cited explicitly in AS 4.3.2, in the teacher’s notes in relation to \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\), making this very much an objective 3 question, linking concepts across the syllabus.
SN2 was commonly given but the mechanism in (ii) was exceptionally poorly answered in this session. In particular, the transition state was rarely drawn, and clearly candidates were not prepared for organic reaction mechanisms, even though there are only a few such examples on the syllabus as a whole.

