| Date | May 2013 | Marks available | 2 | Reference code | 13M.2.sl.TZ1.8 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Deduce | Question number | 8 | Adapted from | N/A |
Question
Ethene belongs to the homologous series of the alkenes.
A bromoalkane, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\), reacts with a warm, aqueous sodium hydroxide solution, NaOH.
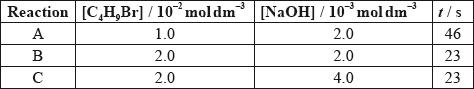
The time taken to produce a certain amount of product using different initial concentrations of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH is measured. The results are shown in the following table.
Outline three features of a homologous series.
Describe a test to distinguish ethene from ethane, including what is observed in each case.
Bromoethane can be produced either from ethene or from ethane. State an equation for each reaction.
State the equation for the reaction of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) with NaOH.
Suggest what would happen to the pH of the solution as the reaction proceeds.
Deduce the effect of the concentration of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH on the rate of reaction.
C4H9Br:
NaOH:
Suggest why warm sodium hydroxide solution is used.
Deduce whether C4H9Br is a primary or tertiary halogenoalkane.
Determine the structural formula of C4H9Br.
Describe, using an equation, how \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) can be converted into \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{B}}{{\text{r}}_{\text{2}}}\).
Explain the mechanism for the reaction in (c) of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) with NaOH, using curly arrows to represent the movement of electron pairs.
Markscheme
same functional group / same general formula;
difference between successive members is \({\text{C}}{{\text{H}}_{\text{2}}}\);
similar chemical properties;
Do not accept “same” chemical properties.
gradually changing physical properties;
adding bromine (water);
ethene: brown/orange to colourless / decolourizes bromine water and
ethane: does not change colour;
OR
adding acidified potassium permanganate solution/\({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\);
ethene: purple to colourless/brown and
ethane: does not change colour;
OR
adding Baeyer’s reagent;
ethene: purple/pink to brown and
ethane: does not change colour;
Do not accept “clear” or “transparent” for “colourless”.
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} + {\text{HBr}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\);
\({{\text{C}}_2}{{\text{H}}_6} + {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}} + {\text{HBr}}\);
Accept structural formulas.
Penalise missing H atoms or incorrect bonds (such as C–HO, C–H2C) in structural formulas only once in the paper.
\({{\text{C}}_4}{{\text{H}}_9}{\text{Br}} + {\text{O}}{{\text{H}}^ - } \to {{\text{C}}_4}{{\text{H}}_9}{\text{OH}} + {\text{B}}{{\text{r}}^ - }\);
Accept NaOH in the equation.
decreases;
C4H9Br:
[C4H9Br] doubles and time halves/rate doubles / rate proportional to [C4H9Br];
Do not accept rate increases when [C4H9Br] increases.
NaOH:
[NaOH] doubles and time/rate does not change / rate independent of [NaOH];
increases rate;
Accept increases number of collisions.
rate depends on \({\text{[}}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br]}}\) only / rate does not depend on \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) / \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction /
first order reaction / if it was primary, reaction would be \({{\text{S}}_{\text{N}}}{\text{2}}\);
tertiary;
Accept ECF.
\({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{3}}}{\text{CBr}}\);
Allow both condensed and full structural formula.
Accept ECF.
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}} + {\text{B}}{{\text{r}}_{\text{2}}} \to {{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{B}}{{\text{r}}_{\text{2}}} + {\text{HBr}}\);
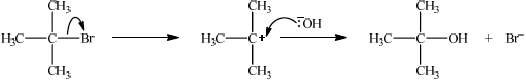
curly arrow showing \({\text{B}}{{\text{r}}^ - }\) leaving;
representation of tertiary carbocation;
curly arrow going from lone pair/negative charge on O in \(^ - {\text{OH}}\) to \({{\text{C}}^ + }\);
Do not allow arrow originating on H in –OH.
formation of \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{3}}}{\text{COH}}\) and \({\text{B}}{{\text{r}}^ - }\);
Accept Br– anywhere on product side in the reaction scheme.
If primary halogenoalkane has been answered in (c)(iii) apply ECF for the mechanism:
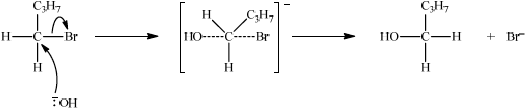
curly arrow going from lone pair/negative charge on O in \(^ - {\text{OH}}\) to C;
Do not allow curly arrow originating on H in –OH.
curly arrow showing \({\text{B}}{{\text{r}}^ - }\) leaving;
Accept curly arrow either going from bond between C and Br to Br in bromobutane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bond;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented.
formation of organic product \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) and \({\text{B}}{{\text{r}}^ - }\);
Accept Br– anywhere on product side in the reaction scheme.
Examiners report
Students had surprisingly difficulties to name the features of a homologous series. Common mistakes were to say SAME chemical or physical properties or same empirical/molecular/structural formula.
Most candidates did well describing the test to distinguish alkanes and alkenes.
The formation of dibromobutane was a common error.
The equation for the reaction of the \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) with NaOH presented no problem.
Some did not realize that pH decreases as NaOH is reacting, often referring as the pH would become more neutral.
Candidates could deduce that the concentration of NaOH does not affect the rate, but could not accurately explain and quantify the relationship between the concentration of C4H9Br and the rate of reaction. Time and rate were often confused.
This was well answered.
Very few candidates could relate rate information to deduce that \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) was tertiary.
The structural formula was generally gained by ECF.
Students did not have problems with the equation.
Mechanism with curly arrows was done very poorly, students confused \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms, drew arrows that did not show clearly origin and end or did not draw any arrow at all.

