| Date | May 2012 | Marks available | 3 | Reference code | 12M.2.sl.TZ2.1 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Calculate | Question number | 1 | Adapted from | N/A |
Question
Hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}}\), releases oxygen gas, \({{\text{O}}_{\text{2}}}{\text{(g)}}\), as it decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
\({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of hydrogen peroxide solution was placed in a boiling tube, and a drop of liquid detergent was added to create a layer of bubbles on the top of the hydrogen peroxide solution as oxygen gas was released. The tube was placed in a water bath at 75 °C and the height of the bubble layer was measured every thirty seconds. A graph was plotted of the height of the bubble layer against time.
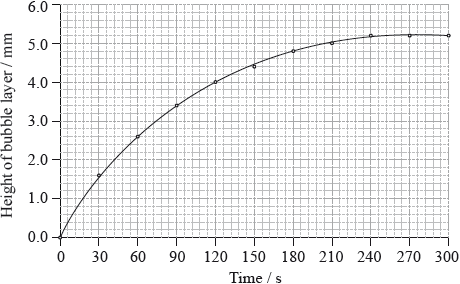
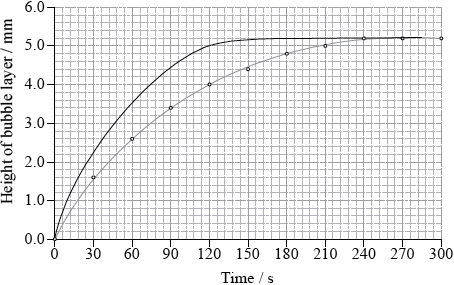
The experiment was repeated using solid manganese(IV) oxide, \({\text{Mn}}{{\text{O}}_{\text{2}}}{\text{(s)}}\), as a catalyst.
The decomposition of hydrogen peroxide to form water and oxygen is a redox reaction.
Explain why the curve reaches a maximum.
Use the graph to calculate the rate of decomposition of hydrogen peroxide at 120 s.
(i) Draw a curve on the graph opposite to show how the height of the bubble layer changes with time when manganese(IV) oxide is present.
(ii) Explain the effect of the catalyst on the rate of decomposition of hydrogen peroxide.
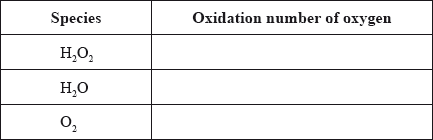
(i) Deduce the oxidation numbers of oxygen present in each of the species below.
(ii) State two half-equations for the decomposition of hydrogen peroxide.
Oxidation:
Reduction:
Markscheme
reaction is complete / all hydrogen peroxide/reactant is used up / no more bubbles are being produced / layer of bubbles is constant / OWTTE;
correctly drawn tangent to the graph at 120 s;
rate = gradient of the tangent to the graph at 120 s / \({\text{rate}} = \frac{{6.0 - 2.0}}{{240 - 0}}\);
\( = 10.017{\text{ mm}}\,{{\text{s}}^{ - 1}}\);
Accept answers in the range 0.014 to 0.020 mm\(\,\)s–1.
Units required for M3.
(i) any line which shows the height of the bubbles increasing much faster;
(ii) catalyst provides an alternative reaction pathway/mechanism with a lower activation energy;
more molecules/particles have energy greater than or equal to the activation energy / OWTTE;
Accept alternative response which refers to mechanism of heterogeneous catalysts.
(i) 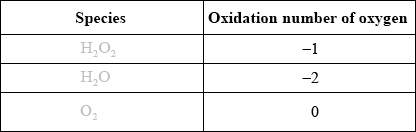
Award [2] for three correct.
Award [1] for two correct.
(ii) Oxidation:
\({{\text{H}}_2}{{\text{O}}_2} \to {{\text{O}}_{\text{2}}} + 2{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - }\);
Reduction:
\({{\text{H}}_2}{{\text{O}}_2} + {\text{2}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - } \to {\text{2}}{{\text{H}}_2}{\text{O}}\);
Examiners report
Part (a) was well answered.
Part (b) was either right or wrong. Few candidates drew the tangent, many being satisfied with a gradient of 4.0/120. Although a number of G2s commented on the “unusual” units of the rate (\({\text{mm}}\,{{\text{s}}^{ - 1}}\)) this did not seem to be an issue for the candidates.
In (c)(i), the line was usually correctly drawn although a significant minority drew it below the original. In (c)(ii), having stated that the catalyst provides a lower activation energy, candidates rarely explained that “more molecules/particles have energy greater than or equal to the activation energy”, many muddling the answer with that appropriate to an elevated temperature.
Most managed the oxidation numbers in (d)(i) although there were some rather curious answers for \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\). There were very few correct answers to the oxidation and reduction half equations in (ii) and this question discriminated the best candidates.

