| Date | May 2015 | Marks available | 2 | Reference code | 15M.2.sl.TZ2.5 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Explain | Question number | 5 | Adapted from | N/A |
Question
When nitrogen gas and hydrogen gas are allowed to react in a closed container, the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\;\;\;\;\;\Delta H = - 92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst. Explain the effect of a catalyst on the rate of reaction.
Sketch the Maxwell–Boltzmann energy distribution curve for a reaction, labelling both axes and showing the activation energy with and without a catalyst.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted-Lowry theory.
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline an experiment and its results which could be used to distinguish between a strong base and a weak base.
Markscheme
rates of forward and reverse reactions are equal / opposing changes occur at equal rates;
the concentrations of all reactants and products remain constant / macroscopic properties remain constant;
closed/isolated system;
Accept “the same” for “equal” in M1 and for “constant” in M2.
\(({K_{\text{c}}} = )\frac{{{{{\text{[N}}{{\text{H}}_3}{\text{(g)]}}}^2}}}{{{\text{[}}{{\text{N}}_2}{\text{(g)]}} \times {{{\text{[}}{{\text{H}}_2}{\text{(g)]}}}^3}}}\);
Ignore state symbols.
Concentration must be represented by square brackets.
The volume of the container is increased:
position of equilibrium shifts to the left/reactants and fewer moles of gas on the right hand side/pressure decreases / OWTTE;
Ammonia is removed from the equilibrium mixture:
position of equilibrium shifts to the right/products and [NH3] decreases so [N2] and [H2] must also decrease to keep Kc constant
OR
position of equilibrium shifts to the right/products and rate of reverse reaction decreases / OWTTE;
Award [1 max] if both predicted changes are correct.
Do not accept “to increase [NH3]” or reference to LCP without explanation.
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction;
Accept “energy difference between reactants and transition state”.
rate increases;
more effective/successful collisions per unit time / greater proportion of collisions effective;
alternative pathway and a lower activation energy
OR
lowers activation energy so that more particles have enough energy to react;
Do not accept just “lowers/reduces the activation energy”.
Accept “provides a surface for reacting/reactants/reaction”.
Curve showing:
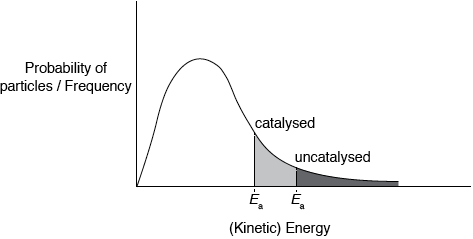
general shape of Maxwell-Boltzmann energy distribution curve and labelled y-axis: probability of particles / frequency and labelled x-axis: (kinetic)energy;
Curve must begin at zero and must not cut the x-axis on the RHS.
Accept number/fraction/proportion of particles for y-axis label, but do not accept amount or just particles.
correct position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
Shading shown in the diagram is not required for the marks.
(i) slower rate / OWTTE;
uneconomic / OWTTE;
(ii) high cost for building/maintaining plant / high energy cost of compressor /OWTTE;
Do not accept “high pressure is expensive” without justification.
Accept high pressure requires high energy.
electron pair donor;
Accept lone pair donor.
proton acceptor and partially/slightly ionized;
Accept “proton acceptor and partially/slightly dissociated”.
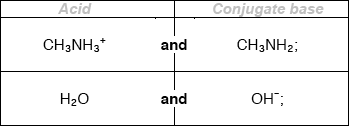
Award [1 max] for two correct acids OR two correct conjugate bases.
solutions of equal concentration;
pH measurement/UIP;
strong base has higher pH;
OR
solutions of equal concentration;
electrical conductivity measurement;
strong base has higher electrical conductivity;
OR
solutions of equal concentration;
temperature difference in neutralization reaction with a strong acid;
strong base has a greater temperature difference;
Accept reverse arguments for observations.
Examiners report
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.

