| Date | May 2017 | Marks available | 2 | Reference code | 17M.2.hl.TZ1.1 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Determine | Question number | 1 | Adapted from | N/A |
Question
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
The general form of the rate equation is:
Rate = [H3CCOCH3(aq)]m × [I2(aq)]n × [H+(aq)]p
The reaction is first order with respect to propanone.
Suggest how the change of iodine concentration could be followed.
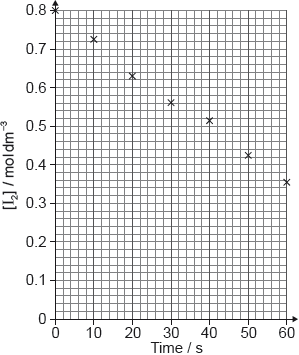
A student produced these results with \([{{\text{H}}^ + }] = 0.15{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Propanone and acid were in excess and iodine was the limiting reagent. Determine the relative rate of reaction when \([{{\text{H}}^ + }] = 0.15{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
Determine the relationship between the rate of reaction and the concentration of acid and the order of reaction with respect to hydrogen ions.
When the concentration of iodine is varied, while keeping the concentrations of acid and propanone constant, the following graphs are obtained.
Deduce, giving your reason, the order of reaction with respect to iodine.
When the reaction is carried out in the absence of acid the following graph is obtained.
Discuss the shape of the graph between A and B.
Markscheme
use a colorimeter/monitor the change in colour
OR
take samples AND quench AND titrate «with thiosulfate»
Accept change in pH.
Accept change in conductivity.
Accept other suitable methods.
Method must imply “change”.
[1 mark]
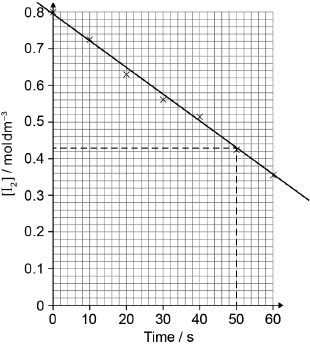
best fit line
relative rate of reaction \( = \ll \frac{{ - \Delta y}}{{\Delta x}} = \frac{{ - (0.43 - 0.80)}}{{50}} = \gg {\text{ }}0.0074/7.4 \times {10^{ - 3}}\)
Best fit line required for M1.
M2 is independent of M1.
Accept range from 0.0070 to 0.0080.
[2 marks]
Relationship:
rate of reaction is «directly» proportional to [H+]
OR
rate of reaction \(\alpha \) [H+]
Order of reaction with respect to [H+]:
first
Accept "doubling the concentration doubles the rate".
Do not accept “rate increases as concentration increases”.
[2 marks]
zero order
rate of reaction is the same for all concentrations of iodine
Accept “all graphs have same/similar gradient”.
[2 marks]
slow rate of reaction which gradually increases
as H+ ions are produced «to catalyse the reaction»
OR
reaction is autocatalytic
M1 should mention “rate of reaction”.
[2 marks]

